Abstract
We explore phorophyte suitability for germination and establishment of the epiphytic orchid, Psychilis kraenzlinii. We found that the orchid grows on a subset of the available tree species and shows preference for the endemic Machaonia portoricensis (Rubiaceae). The orchid preferred trees with smoother bark, high water holding capacity and low water retention capacity. Microclimatic conditions under which embryos began pre-germination stages mirrored that of the adult orchid, but germination did not, suggesting that suitable germination sites are not necessarily the best sites for later stages of development.
Keywords: community dynamics; epiphytes; germination; phorophytes; population dynamics; Orchidaceae
Resumen
Exploramos la utilización de árboles y los patrones de germinación de la orquídea epífita, Psychilis kraenzlinii. Encontramos que P. kraenzlinii crece en un subconjunto de las especies de árboles disponibles y muestra preferencia por la endémica Machaonia portoricensis. La orquídea prefiere árboles con corteza lisa y alta capacidad de sostener agua y baja capacidad de retención de agua. Las condiciones microclimáticas bajo las cuáles los embriones empienzan etapas pre-germinacíon, reflejan los de la orquídea adulta, pero las condiciones bajo las cuáles los embriones llegan a etapas de germinación no. Lo que sugiere que los sitios de germinación adecuados no son necesariamente los lugares donde mejor se producirá el desarrollo a etapas más avanzadas de la germinación.
Palabras clave: dinámica comunitaria; dinámica poblacional; epífitas; forófitos; germinación; Orchidaceae
Introduction
Orchids are generally characterized by small, scattered populations (Ackerman 1986, Tremblay 1997), making many species vulnerable to deforestation, habitat fragmentation, and illegal collection (Adhikari & Fischer 2011). Factors of paramount importance that limit orchid abundance and distribution are believed to be pollinator availability and its influence on seed production (Ackerman et al. 1996), and OMF availability, which may be microsite-limited (Izuddin et al. 2019a, 2019b, Otero & Flanagan 2006). Because of their highly variable and important symbiotic relationships, orchid conservation and management strategies might need to be developed individually for genera or even species and include the entire communities in which they occur (Fay 2018, Phillips et al. 2020, Rasmussen et al. 2015).
Approximately 70% of orchid species are epiphytes, accounting for approximately 72% of epiphyte species in the world (Gentry & Dodson 1987, Gravendeel et al. 2004). Epiphytic orchid conservation and management techniques may include the protection of suitable and existing phorophytes, as well as planting new ones (Adhikari & Fischer 2011). While our knowledge of the relationship between epiphytes and phorophytes has advanced, relatively few epiphytic species have been studied in detail (e.g., Benzing 1990, Gowland et al. 2011, Sáyago et al. 2013, Zotz et al. 2021) yet we do know that phorophyte specificity is rare. Still, some degree of preference is commonly found within sites (Gowland et al. 2011, Laube & Zotz 2006, Migenis & Ackerman 1993, Sulit 1950, 1953, Trapnell & Hamrick 2006, Tremblay et al. 1998, Wagner et al. 2015). On the other hand, among sites, Hietz & Hietz-Seifert (1995) found epiphyte community composition was more closely associated with elevation rather than the availability of particular phorophyte species.
The epiphytic environment is in constant change, as host trees grow and age. Changes in the crown of the tree, for example, affect radiation, temperature, and humidity along the entire tree (Benzing 1979, 2004, Rasmussen & Rasmussen 2018). Physical and chemical characteristics of the bark can also affect the presence of mycorrhizal fungi, probability of seed attachment, germination and/or establishment (Frei & Dodson 1972, Sáyago et al. 2013, Siaz-Torres et al. 2021). Bark traits that may affect the presence of epiphytes include rugosity (which might be affected by age), water storage capacity (that could be affected by bark rugosity), pH, and secondary metabolites (Adhikari & Fisher 2011, Frei 1973, Frei & Dodson 1972, Migenis & Ackerman 1993, Sáyago et al. 2013, Siaz-Torres et al. 2021, Timsina et al. 2016). Here we study an epiphytic orchid endemic to Puerto Rico, Psychilis kraenzlinii (Bello) Sauleda. The genus Psychilis is composed of 15 epiphytic species that are distributed among Hispaniola, Puerto Rico, the US and British Virgin Islands, and Northern Lesser Antilles (Ackerman & Collaborators 2014, Sauleda 1988). The genus is severely understudied, lacking conservation and management strategies for most species. The present study uses a population of P. kraenzlinii in the Susúa State Forest as a model to explore the relationship of orchids with their phorophytes (González-Orellana et al. 2022).
First, we asked if P. kraenzlinii shows phorophyte preferences and whether these preferences correspond to where germination occurs most frequently. Like the closely related species, Psychilis monensis Sauleda (Otero et al. 2007), Psychilis krugii (Bello) Sauleda (Ackerman et al. 1989), and Psychilis truncata (Cabrera-García et al. 2023), we expected P. kraenzlinii will not be host-specific and would instead show a preference for a subset of the available phorophyte species. Using different taxa as phorophytes could be advantageous for epiphytes as the epiphytic habitat is a stressful one and constantly changing (Benzing 1979, Trapnell & Hamrick 2006, Tremblay et al. 2006). We also hypothesized that seed germination would mirror phorophyte associations of established epiphytic orchids since one may assume that, like terrestrial orchids, the presence of an established orchid can be an indicator of suitable environmental conditions and OMF availability (Jacquemyn et al. 2007, McCormick et al. 2016, Petrolli et al. 2021).
Secondly, we explored other factors that may affect germination and establishment of P. kraenzlinii in the Susúa State Forest. We measured Water Storage Capacity (WSC) and bark roughness of phorophytes to determine whether these traits differed among phorophyte species and between trees with and without the orchid. Epiphytes are prone to be water stressed (Benzing 2004). Rough-barked trees are generally colonized more frequently by epiphytes (Callaway et al. 2002) perhaps due to better water retention capacity or because seeds more readily attach to them (Adhikari & Fisher 2011, Timsina et al. 2016). Consequently, we expected to find higher seed germination rates and more orchids on phorophytes with high roughness and water retention capacity.
Finally, we used the germination stages of seeds as a proxy for the presence of orchid mycorrhizal fungi (OMF) on phorophytes. Orchid seed imbibition must occur before mycorrhizal infection (Bidartondo 2005, Rasmussen 1995). Imbibition is indicated when the embryo swells and breaks the seed testa (Brandner 2005). Afterwards, fungal infection can occur, which leads to the uptake of nutrients by the plant making cell division and growth possible (Arditti 1992, Rasmussen 1995). Hence, we assumed that seeds that reached germination were infected by their OMF. If orchids and their OMF share similar niche requirements (Izuddin et al. 2019a, 2019b), then we expect that protocorm formation will be more likely on phorophyte species that have a higher occurrence of established orchids.
Materials and methods
Study system-. Psychilis kraenzlinii is a rewardless, self-incompatible epiphyte that produces long, erect peduncles topped by racemes of sequentially produced red-carmine flowers (Ackerman & Collaborators 2014). Populations flower and set fruit throughout the year, but studies done on the closely related species P. krugii and P. monensis (Ackerman et al. 1989, Aragón & Ackerman 2004, Otero et al. 2007) suggest that peak flowering occurs from April through July. Psychilis kraenzlinii resides in the limestone hills and margins of mangrove swamps on the north side of the island, and in tropical moist forest regions on the southern slopes of the Cordillera Central. Although it is widely distributed across the island of Puerto Rico, many populations are now believed to be extinct due to habitat destruction through anthropogenic activities such as deforestation, limestone mining and urbanization. Populations have also been severely affected by legal and illegal collection. There are no published ecological studies on this species, but it was classified as vulnerable by Miller et al. (2013).
Study site-. Susúa State Forest is a Natural Reserve under the jurisdiction of the Department of Natural and Environmental Resources of Puerto Rico. The forest occupies about 13 km2 across the municipalities of Yauco and Sabana Grande (18°04’14.6” N 66°54’23.4” W), on the southwestern slope of the Cordillera Central (Departamento de Recursos Naturales y Ambientales 2015). This moist forest is characterized by serpentine and volcanic soils, and has 157 tree species, 16 of which are classified as rare or endangered. Average annual precipitation is 1413 mm and average temperature is 23.9°C. Before the establishment of the State Forest in 1935, the area was almost completely deforested for agriculture, wood products, and minerals (DRNA 2015). The combination of secondary growth and nutrient-poor ultramafic soils has resulted in a mostly evergreen forest comprised of slender trees averaging 12 m tall, with a light canopy (Miller & Lugo 2009).
Phorophyte Specificity Assessment-. The study site consisted of a single population in one area of the forest. To cover as much of the area as possible, we established four 15 × 5 m plots at approximately 5 m from each other. Trees and shrubs inside the plots were identified, DBH was measured, and we noted if they had P. kraenzlinii. All P. kraenzlinii plants inside plots were tagged. Given the small sample size, for the analysis we filtered the data and kept only the tree species that had a frequency higher than 3%. We applied a Fisher’s Exact Test to see if there was a relationship between each tree species and the presence of the orchid.
Phorophyte Physical Characteristics-. The two physical characteristics of the bark that we considered were Water Storage Capacity (WSC) and Fissuring Index (FI), both of which influenced orchid host tree preferences in Mexico (Zarate-García et al. 2020). The tree species from which we collected bark data were chosen based on the Phorophyte Specificity Assessment described previously to create a gradient from positive to negative relationship as follows: Machaonia portoricensis Baill. (Rubiaceae), Phyllanthus cuneifolius (Britton) Croizat (Phyllanthaceae), Ouratea littoralis Urb. (Ochnaceae), Rondeletia inermis (Spreng.) Krug & Urb. (Rubiaceae), Tabebuia haemantha (Bertol. Ex Spreng.) DC. (Bignoniaceae), Swietenia mahagoni (L.) Jacq. (Meliaceae) and Coccoloba microstachya Willd. (Polygonaceae). Because most orchids grew attached to phorophytes at a height below 0.75 m, we collected bark samples no higher than that. When possible, half the samples were collected near the roots of adult orchids, and half from trees where the orchid was absent.
Water Storage Capacity Assessment (WSC)-. To measure WSC we adapted the methodologies described by Callaway et al. (2002) and Zarate-García et al. (2020). In the laboratory, samples were cut to approximately 1 cm2 and dried in an oven at 40°C. Drying time fluctuated between 24 h and 72 h for each species, since bark rugosity and thickness of the bark varies among species. After drying, samples were weighed to obtain dry mass and their length, width, and thickness was measured with a caliper to calculate volume. We then submerged the samples in water treated with Triton X-100 for 30 min, allowed to drip for a minute and weighed to obtain wet mass. Finally, they were left to air dry for 24 h, after which they were weighed again to obtain held mass. Water Holding Capacity (WHC) and Water Retention Capacity (WRC) were calculated per volume of the sample as defined by Callaway et al. (2002):
Where WHC refers to how much water adheres to the bark immediately after it becomes wet (cohesion), while WRC refers to how much water adheres and remains within the bark after 24h of becoming wet.
We collected 187 samples of bark from which 74 were from trees with P. kraenzlinii. Samples from S. mahagoni were collected only from trees without the orchid because it rarely served as a host to P. kraenzlinii in our study site. The number of samples per species is described in Appendix 1. We applied a Kruskal-Wallis Test to determine if WSC was different among species, and a Mann-Whitney U to evaluate if differences in indices between trees with and without P. kraenzlinii where significantly different. If significant differences were found, then a Conover-Iman Pair-Wise Comparison was applied to detect which species had a significant effect. Intraspecific differences between trees with and without P. kraenzlinii could only be evaluated by removing S. mahagoni, since no data for trees with the orchid was surveyed. The following trees species were excluded when evaluating the effect of WRC because no differences was observed between trees with and without orchids and all values were 0.0 g/mm3 (M. portoricensis, R. aculeata, and R. inermis).
Fissuring Index Assessment-. We used two methods for preparing bark to calculate a Fissuring Index (FI). In the first method, the bark samples were dried and cleaned carefully with alcohol (Zarate-Gracía et al. 2020). The second procedure was to use untreated, fresh bark samples. To test which was the better method, we took 3 samples from 3 trees of 3 species growing on campus of University of Puerto Rico, Río Piedras. We selected flaky bark from S. mahagoni, smooth bark from Ficus macrocarpa L.f. (Moraceae), and rough bark from Tabebuia heterophylla (DC.) Britton (Bignoniaceae). All samples were photographed, and photos were cropped to cover 1 cm2. Photos were uploaded into R where they were transformed into gray scale and then into binary (black and white) images using the package imager v.45.2 (Barthelme et al. 2023). We counted the number of black (fissured bark) and white (non-fissured bark) pixels, and with these data calculated the fissuring index of Zarate-García et al. (2020):
We compared the FI measured by each method using a Mann-Whitney U Test that revealed no significant difference (p > 0.05) between the two methodologies. Since there was no significant difference, we decided to use fresh samples for the P. kraenzlinii work. The fissuring index is a measure of the texture of the surface of the bark (fissures, bumps, and irregularities) (See Zarate-García et al. 2020). The higher the fissuring index, the less rough or irregular the surface of the bark.
We collected 194 samples of bark of which 83 were from trees with P. kraenzlinii. The number of samples per species is described in Appendix 2. Samples from R. aculeata and S. mahagoni were only from trees without the orchid, since finding the orchid growing on these species was rare. A Kruskal-Wallis Test was applied to see if FI was different among species. If a significant difference was found, a Conover-Iman Pair-Wise Comparison was applied to know which species had a significant effect. Mann-Whitney U test was used to detect significant differences between trees with and without the orchid, both in general and within each species of phorophytes.
In situ seed germination-. Seed packets were built by sewing 3 × 5 cm nylon plankton netting fabric with mesh size 45 µ (an adaptation of Zi et al. 2014). A sample of the seeds from each fruit was tested for viability with tetrazolium chloride (TTC). Once viability was confirmed, 200-230 seeds were placed inside packets which were then secured to tree bark with gutter mesh (Khamchatra et al. 2016). On each of six phorophyte species selected, we placed one packet on 20 trees, and on Coccoloba microstachya and Machaonia portoricensis, we placed one packet on 30 trees in May and June 2021. After 7 months we collected the packets and examined them under a dissecting microscope in the laboratory. We then created a developmental stage classification system for P. kraenzlinii based on Stenberg & Kaine (1998) and Brandner (2005) (Table 1).
Description of embryo development stages of Psychilis kraenzlinii grown in situ developed by the authors based on Stenberg & Kaine (1998) and Brandner (2005). Stages 1 and 2 are early development, whereas Stages 3 and 4 are considered the first germination stages, since the protocorm is formed.
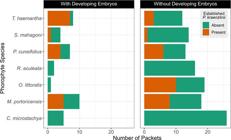
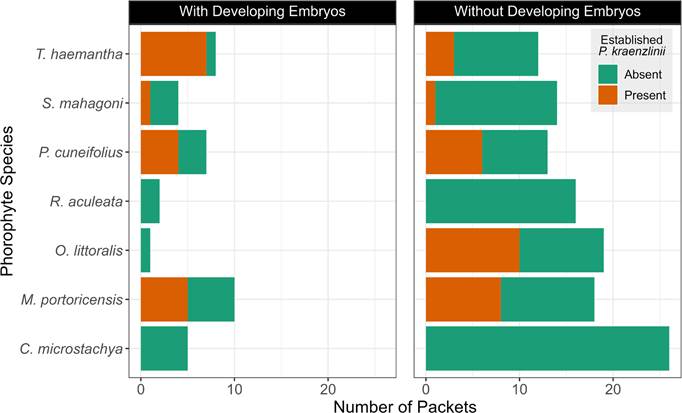
We recovered 174 seed packets from the forest of which 51 were on trees with P. kraenzlinii. (The imbalance between numbers of trees with and without P. kraenzlinii in C. microstachya, R. aculeata, R. inermis and S. mahagoni is because P. kraenzlinii rarely grew on them.) Packets on C. microstachya and R. aculeata were only placed on trees without the orchid. Only 6 packets were placed on trees with the orchid on R. inermis, and only 2 packets were placed on trees with the orchid on S. mahagoni (Appendix 3).
We investigated the influence of the presence of P. kraenzlinii and/or the phorophyte species on the number of packets with developing seeds by applying a Generalized Linear Model (GLM) with a binomial distribution. Odds ratios were calculated to measure the association between the presence of the orchid or the species of phorophyte and the number of packets with developing seeds. Odds ratio is used to measure the strength of an association between an observation and an outcome, where an odds ratio equal to 1 suggests no association, odds ratio greater than 1 suggests positive association, and odds ratio less than 1 suggests a negative association (see Szumilas 2010). To detect an association between the presence of P. kraenzlinii and orchid developing seeds, we excluded data from R. aculeata and C. microstachya because no trees with the orchid were available to place packets for comparisons. Packets placed on R. inermis were removed from all analyses related to embryo development because there was no development on R. inermis.
To analyze the influence of the presence of P. kraenzlinii and/or the phorophyte species on the percentage of developing seeds, we applied a Generalized Linear Model (GLM) using a nonbinomial distribution. Odds ratios were calculated to measure the association between the presence of the orchid or the species of the phorophyte and the percentage of developing seeds (Szumilas 2010). Then we asked whether some phorophyte species had a higher percentage of seeds at each developmental stage and used a Kruskal-Wallis test for each germination stage among the different phorophyte species, and a Mann-Whitney test to compare trees with and without an established P. kraenzlinii. If a difference was detected when using the Kruskal-Wallis, then a Conover-Iman test was applied to identify which species were significantly different. This would suggest that some tree species were a better substrate for seeds to develop than others. Finally, we explored if the presence of P. kraenzlinii or the species of the phorophyte could predict the presence of OMF by using the germination stages as a proxy for the presence of OMF on a GLM with binomial distribution. Odds ratio for the association between the presence of P. kraenzlinii or the phorophyte species and the presence of the OMF were calculated (Szumilas 2010).
Results
Phorophyte Utilization Assessment-.Size of the trees: The plots had 568 trees belonging to at least 27 species. Most trees in our plots had a DBH less than 3.0 cm (x̄ = 2.5 cm, Q0.25= 1.3 cm, Q0.5 = 1.9 cm, Q0.75 = 2.9 cm), and trees with P. kraenzlinii growing on them had a larger mean (x̄ = 2.7 cm) than the median ( Q0.5 = 2.0 cm). We tagged 117 P. kraenzlinii growing on 13 (48%) tree species (Table 2). Most orchids grew less than 0.75 m above ground (x̄ = 0.42 m, Q0.25= 0.22 m, Q0.5 = 0.36 m, Q0.75 = 0.58 m).
Phorophyte and orchid association: There was a significant association between the presence of Psychilis kraenzlinii and the species of tree (Fisher Exact Test, p < 0.01, Monte Carlo Simulation = 2000). A Fisher’s pairwise comparison of pooled plot data revealed significant differences between Machaonia portoricensis and Coccoloba microstachya (p < 0.005, Fig. 1), where the former has a higher number of orchids than the latter. There were more orchids growing on M. portoricensis than expected if the presence of the orchid among phorophyte species was random. Conversely, there were fewer orchids growing on C. microstachya than expected.
A. Proportion of trees of each phorophyte species harboring Psychilis kraenzlinii (unidentified phorophytes not included).
Water Storage Capacity-. Water Holding Capacity: We found WHC to be significantly different among phorophyte species (WHC, Kruskal-Wallis test: Χ 2 = 82.62, df = 7, p < 0.005,), but not between trees with or without the orchid (Mann-Whitney: U = 4074.5, p = 0.77). The Conover-Iman Pairwise test for the WHC (Appendix 4) showed that M. portoricensis has the highest WHC, being significantly different from all species except R. inermis. The lowest WHC is that of O. littoralis, which was significantly different from all species but S. mahagoni. Among trees occupied by P. kraenzlinii, T. haemantha had a significantly higher WHC, whereas Coccoloba microstachya and M. portoricensis had a significantly lower WHC (Appendix 5).
Water Retention Capacity: We discovered differences in WRC among phorophyte species (Kruskal-Wallis test: Χ2 = 43.22, df = 7, p < 0.005), but not between trees with or without the orchid (Mann-Whitney: U = 4560.5, p = 0.22). The Conover-Iman Pairwise Comparison test for WRC (Appendix 4) showed that the highest WRC was that of S. mahagoni, and it was significantly different from all other species. Randia aculeata, R. inermis and M. portoricensis have WRC of <0.01 g/mm3 which was significantly lower than S. mahagoni, T. haemantha, and O. littoralis. Phyllanthus cuneifolius has a WRC significantly higher than M. portoricensis and R. inermis, and although higher than R. aculeata, this last difference is not significant. When comparing trees of each species with and without the orchid, we also found no statistically significant differences (Appendix 5).
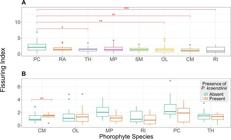
Fissuring Index Assessment-. Phorophyte species differed significantly in FI (Kruskal-Wallis: Χ 2 = 15.07, df = 7, p = 0.04). Phyllanthus cuneifolius had the higher FI, but the difference was only significant when compared to C. microstachya, O. littoralis, R. inermis, or T. haemantha. We found that P. cuneifolius and R. inermis had the greatest variation in FI among species, but in general P. cuneifolius had a higher FI while R. inermis had the lowest (Appendix 4, Fig. 2A). We observed that 3 out of the top 4 species with higher fissuring index also had high WHC, while 3 out of the 4 species with lower FI had higher WRC.
Significant differences exist in the fissuring index between trees with and without the orchid (Mann-Whitney: U = 5.29, df = 1, p = 0.02). Trees with P. kraenzlinii had a significantly lower FI. When evaluating this relationship for each species, the trend repeated within most, but it was only significant for M. portoricensis (Mann-Whitney: U = 208, p < 0.005, Fig. 2B).
In situ seed germinations-. Of the 174 recovered packets, only 37 (21%) contained developing seeds. Of the 37 packets with developing seeds, 20 (54%) were near an established P. kraenzlinii. Of the 118 packets without developing seeds, 90 (76%) were on trees without an established orchid. A chi-square revealed that the number of packets with developing seeds near an established orchid is not significantly higher than if an established orchid was not present (Χ 2 = 0.24, df = 1, p = 0.62). The best model to explain the number of packets with and without developing seeds was a binomial one where C. microstachya was placed as the intercept (Model A in Table 3). The odds of a packet with developing seeds were higher near an established orchid (OR = 1.4) and if it was located on T. haemantha (OR = 2.2) or M. portoricensis (OR = 1.9, Model A in Table 4, Fig. 3).
Factors associated with the effect of phorophyte species on seed germination. Analyses are based on coefficients generated by Generalized Linear Models. Model A: Negative Binomial Model for the effect of phorophyte species and the presence of an established Psychilis kraenzlinii on the number of packets with germinated seeds. Model B: Negative Binomial Model for the effect of phorophyte species on the percent developing seeds. Model C: Effect of phorophyte species and presence of established Psychilis kraenzlinii over the presence of Orchid Mycorrhizal Fungi (OMF) when using embryo development stages as a proxy for OMF presence. SE = Std. Error.
Association among phorophyte species, presence of adult Psychilis kraenzlinii, and germination success based on Odds Ratios (OR) calculated for each Generalized Linear Model with 95% Confidence Intervals (CI). Model A: Negative Binomial Model for the effect of phorophyte species and the presence of an established Psychilis kraenzlinii on the number of packets with germinated seeds. Model B: Negative Binomial Model for the effect of phorophyte species on the percent developing seeds. Model C: effect of phorophyte species and presence of established Psychilis kraenzlinii over the presence of Orchid Mycorrhizal Fungi (OMF) when using embryo development stages as a proxy for OMF presence.
The number of packets with and without developing seeds on each phorophyte species and whether they were placed near an established Psychilis kraenzlinii or not.
We observed 228 developing seeds among all packets, 121 of which were in packets near a P. kraenzlinii. The proportion of developing seeds per packet near an established orchid was significantly higher than that of packets in trees without the orchid (Mann-Whitney test, U = 2049.5, p = 0.02). The development stage of the seeds was evaluated according to our classification scheme (Table 1). We found 85 (40%) seeds in Stage 1, 76 (35%) in Stage 2, 35 (16%) in Stage 3, and 7 (3%) in Stage 4. No germination was noted from packets on R. inermis. The distribution of developing seeds in different stages among phorophytes is shown in Figure 4.
Mean percent of developing seeds per packet and their development stage on phorophytes with and without an established Psychilis kraenzlinii.
The best model to explain the percentage of developing seeds was a negative binomial GLM with the phorophyte species as predictor variable (Model B in Table 3). The presence of an established P. kraenzlinii did not have a significant effect on the percentage of such seeds. Among all phorophytes, M. portoricensis is the only species with a significant and positive effect on the percentage of developing seeds, while C. microstachya is the intercept with a significant and negative effect. The odds of P. kraenzlinii developing on C. microstachya are near zero, while the odds for developing on M. portoricensis are 5.22. Other species with high odds of P. kraenzlinii seeds developing are S. mahagoni (OR = 4.18), T. haemantha (OR = 3.76), and P. cuneifolius (OR = 3.10, Model B in Table 4).
When comparing the percentage of seeds in each germination development stage among phorophytes, O. littoralis, C. microstachya and R. aculeata had significantly more seeds that did not develop (stage 0) than T. haemantha; O. littoralis and R. aculeata also had more seeds in stage 0 than P. cuneifolius (Conover-Iman: p < 0.05) (Appendix 6). Conversely, T. haemantha had significantly more seeds that went through imbibition (stage 1) than C. microstachya, O. littoralis, and R. aculeata (Conover-Iman: p < 0.05) (Appendix 6). Tabebuia haemantha and P. cuneifolius had significantly more seeds whose embryo swelled to the point of breaking the testa (stage 2) than C. microstachya and O. littoralis; T. haemantha also had more seeds in stage 2 than M. portoricensis, and P. cuneifolius had more than R. aculeata (Conover-Iman: p < 0.05, Appendix 6). There was no difference in the occurrence of stage 3 (Kruskal-Wallis: Χ 2= 4.16, df = 6, p = 0.66) and 4 (Kruskal-Wallis: Χ 2 = 8.36, df = 6, p = 0.21) among phorophytes. Trees with an established orchid had significantly fewer non-germinated seeds (Mann-Whitney: U = 2997.5, p < 0.005), and significantly more seeds in stage 1 (Mann-Whitney: U = 2048.5, p = 0.006), stage 2 (Mann-Whitney: U = 1891.5, p < 0.005), and stage 3 (Mann-Whitney: U = 2228.5, p = 0.04). There was no difference in the percentage of seeds on stage 4 between trees with and without an established P. kraenzlinii.
The best model to predict if the OMF was present or not was a GLM with binomial distribution that had both the phorophyte species and the presence of an established orchid as predictor variables. Coccoloba microstachya had a significant and negative effect over the presence of the OMF (Model C in Table 3). The OMF was 4.7 (OR) times more likely to be found near an established P. kraenzlinii and 3.7 (OR) times more likely to be found on S. mahagoni, irrelevant of whether there was an established orchid or not, according to the odds ratio (Model C in Table 4).
Discussion
We evaluated the phorophyte preferences in a population of Psychilis kraenzlinii and found that they are not randomly distributed among the available tree species in our study population. Furthermore, the best phorophytes for germination are not necessarily the same as those for adults. The best phorophytes for P. kraenzlinii are either rare or endemic species. Nonetheless, the population is healthy and with recruitment, which underscores the importance of continued protection of the forest.
Phorophyte Specificity Assessment-.Migenis & Ackerman (1993) suggested that host preference rather than specificity is common in Puerto Rico and the Neotropics. As for closely related species P. monensis and P. krugii of Puerto Rico (Ackerman et al. 1989, Otero et al. 2007), and P. truncata in the Dominican Republic (Cabrera-García et al. 2023), P. kraenzlinii only grows on a subset of available phorophytes. While it shows highest preference for Machaonia portoricensis, Coccoloba microstachya is the least preferred phorophyte given the abundance of this tree species in the study area. Contrastingly, Otero et al. (2007) found that C. microstachya is a common phorophyte of P. monensis on Mona Island, Puerto Rico. Sanford (1974) suggested that the different usage of phorophytes by an orchid species in different geographical areas was indicative of the importance of the whole habitat instead of only a few factors such as phorophyte species and their characteristics. Thus, the ability of an epiphyte to germinate and develop on a certain tree species, not only depends on climate, habitat, forest structure and characteristics of phorophytes, but also on microsite conditions such as temperature, humidity, microbial symbionts, etc. Otero et al. (2007) noted that the relationship of P. monensis with its phorophyte species is site-dependent and they suggested this was due to water relations. Data for phorophyte usage of P. kraenzlinii in other regions of Puerto Rico are not available, but phorophyte preferences of this species, like that of other epiphytes, might change according to environmental stressors (Sanford 1974, Timsina et al. 2016).
Phorophyte Physical Characteristics-.Variation in WHC and WRC between trees with and without the orchid irrespective of their species, was not significant. However, we found that Machaonia portoricensis, the most preferred phorophyte species, has the highest WHC, but no WRC. Other species on which the orchid was commonly found, Phyllanthus cuneifolius and Rondeletia inermis, also had a high WHC and no WRC. Conversely, C. microstachya, the least preferred phorophyte species, has the second lowest WHC, and a higher WRC. Intermediate conditions do exist. Psychilis kraenzlinii is frequently found on Ouratea littoralis, but unlike other preferred phorophytes (M. portoricensis, P. cuneifolius, R. inermis), it has the second lowest WHC and an intermediate WRC. Nonetheless, in general, preferred phorophytes tend to have high WHC and low WRC. We hypothesize that when it rains, the preferred phorophytes have the capacity to hold more water, giving the opportunity for seeds to go through the imbibition process rapidly. Then, these phorophytes quickly lose water (low WRC), preventing seeds from becoming waterlogged. As the imbibition process must occur before the infection of the OMF (Rasmussen, 1995), preferred phorophytes with high WHC promote rapid imbibition, resulting in ready-to-infect seeds faster than those phorophytes that have low WHC. Seeds growing on phorophytes with low WHC, but high WRC, might take longer to go through the imbibition process and become infected by their OMF, resulting in longer exposure to adverse environmental conditions, pathogens, or grazers. Although O. littoralis seems to share characteristics with the less common phorophytes, it has the lowest WHC. The fact that the orchid is commonly found growing on this species might be explained by the low WHC preventing the seeds from waterlogging, and its high WRC giving the seeds time to go through the imbibition process without desiccating. Wagner et al. (2015) mention that a low WRC might be suitable for epiphytes on a mesic habitat. Hence, in the moist forest of Susúa, low WRC might render smooth barked species good phorophytes for P. kraenzlinii, since water relations may be balanced.
Bark roughness may be associated with water storage capacity (Migenis & Ackerman 1993, Otero et al. 2007, Zarate-García et al. 2020). We observed a trend where species with higher WHC had lower FI (smoother bark), while those species with higher WRC had higher FI (rougher bark). Nonetheless, this association could not be statistically tested with our data. Bark roughness may also help seeds attach to the trunk of trees (Callaway et al. 2002, Siaz-Torres et al. 2020). Hence, it might explain why those phorophytes with low WHC, but high WRC, like O. littoralis, still harbor the orchid. Their roughness promotes attachment, and the crevices might serve as protection to give time for seeds to develop under a low but time-continuous water supplement. This hypothesis is supported by the fact that irrespective of the phorophyte species, trees on which P. kraenzlinii was growing had significantly rougher bark (lower FI) than those trees lacking the orchid. Furthermore, intraspecific differences in FI between trees with and without the orchid was only significant in M. portoricensis, where more orchids were growing on trees with rougher bark (lower FI). This relationship is also present as a non-significant trend among species with smoother bark (R. inermis and P. cuneifolius). Rondeletia inermis superficially appears to have smooth bark, but microscopically the bark appears rough with numerous crevices. Nonetheless, R. inermis behaves as a smooth bark species (high WHC, no WRC) because its bark is thin, unlike other rough-barked species (T. haemantha, O. littoralis, C. microstachya and S. mahagoni) which all have thick spongy bark. The hypothesis that P. kraenzlinii prefers phorophytes with rough bark and high WRC is not supported. It appears that the contrary is true.
In fact, most P. kraenzlinii were found growing at the base of the tree-no higher than 0.75 m from the ground-where humidity is higher and light exposure is lower Petter et al. (2016), likely meaning more water availability. Phorophyte preferences of two dry-forest Psychilis species have also been studied using subjective assessments of bark roughness. Ackerman et al. (1989) found no preference for rough-barked species by P. krugii in Guánica, Puerto Rico, and Otero et al. (2007) discovered that P. monensis on Mona Island was very common on rough-barked Phyllanthus epiphyllanthus, but when they eliminated that phorophyte from their analysis they found no preference for other rough-barked phorophyte species.
Since trees tend to have different pigmentation patterns on their bark, the FI results must be interpreted cautiously. The bark of tree species we studied is not uniformly colored, which may affect the FI results (Fig. 5). Sections of the bark with dark colors such as green and brown could be interpreted by the algorithm as roughness, while light pigmentation like white and pink could be interpreted as smoothness. While we do not yet know whether this is a problem, staining the bark surface to cover such pigmentation may be advisable.
Psychilis kraenzlinii prefers trees with smoother bark (high FI), high WHC and a low WRC. Conversely, Zarate-García et al. (2020) found no clear correlation between FI of phorophytes and the presence of orchids. Furthermore, they did find phorophyte preference was inversely correlated with WHC, while positively correlated with WRC. Bark roughness preferences might be influenced by microsite conditions such as radiation exposure, humidity, and seasonality as well as by the method of attachment used in each stage of the life history of an epiphytic species (Tay et al. 2023). The study sites in Zarate-García et al. (2020), were low coastal forests in the Yucatan Peninsula, Mexico, where mean annual temperature is higher and mean annual rainfall is lower than our study site in Susúa State Forest, Puerto Rico. Environmental conditions and phorophyte phenology at these sites might affect phorophyte preferences of the orchid species studied (Zarate-García et al. 2020). Similarly, Ackerman, Montalvo & Vera (1989) and Otero et al. (2007) found no clear relationship for either P. monensis or P. krugii between phorophyte preference and bark roughness. However, subjective assessments of bark topography, such as the one used by those authors could be misleading (Tay et al. 2023). Guánica State Forest and Mona Island are dry environments with low, open canopies where P. krugii and P. monensis might be exposed to direct sunlight and drought. Phorophyte preferences might be governed by factors such as light exposure, rather than only by phorophyte characteristics. In contrast, the Susúa State Forest is a moist forest with a dense canopy cover that protects orchids against radiation and water evaporation. Hence, orchid germination might be influenced by higher WHC rather than WRC because it promotes rapid germination, reducing the probability of experiencing adverse conditions during early stages of development.
In Situ Seed Germination-. Germination of terrestrial orchids is higher near established plants, which may serve as a beacon of suitable conditions and/or a reservoir of mycorrhizal fungi (Diez 2007, McCormick et al. 2016). In situ germination studies of epiphytic orchids are limited, contrary to the studies of terrestrial orchids, Kartzinel et al. (2013) found that Epidendrum firmum Rchb.f. was dependent on the microclimates of large trees and closed canopies, rather than proximity of conspecific adults. Conversely, Petrolli et al. (2021) found a correlation between OMF community composition with epiphyte root proximity, suggesting that the bark near established orchids likely harbor their OMF. Further evidence of spatial structure was revealed when Petrolli et al. (2022) and Fernández et al. (2023) discovered that epiphytic orchid communities formed modular networks with their OMF. In addition, studies for both terrestrial (Whitman & Ackerman 2015, Jacquemyn et. al. 2007, Jersáková & Malinová 2007) and epiphytic species have suggested that spatial distribution of orchids may be dependent on propagule pressure which is strongest near seed sources (Ackerman et al. 1996).
A higher frequency of seeds in process of germinating was obtained near established orchids. Still, the model (negative binomial GLM) that best explains the data did not include the variable of presence of an established P. kraenzlinii as a predictor. We hypothesized that germination would be higher near established orchids because of a higher propagule pressure, higher probability of OMF availability and appropriate microsite conditions. Our results suggest that germination is more probable near established orchids, supporting our hypothesis, but that the phorophyte species has a stronger effect on the percentage of developing seeds. According to the model, P. kraenzlinii has a significantly higher probability of developing on M. portoricensis, and a significantly lower probability of developing on C. microstachya. Hence, the patterns of P. kraenzlinii seeds that are ready to undergo germination tend to mirror that of the distribution of established orchids in the study site.
Germination development stages reached by seeds in packets on different phorophyte species varied. The percentage of non-germinated seeds (Stage 0) was highest on O. littoralis and R. aculeata. Those that reached Stages 1 and 2 were more common on T. haemantha and P. cuneifolius. Nonetheless, the later stages (Stage 3 and 4) showed no difference among phorophyte species so that early-stage success is not necessarily indicative of success in reaching later stages. In fact, we found no significant difference among phorophyte species in the presence of OMF. Remarkably, the highest probability of having OMF (as evidenced by germination to at least stage 4) was S. mahagoni, a species where established orchids are rare to find. Furthermore, R. aculeata, a species largely unoccupied by P. kraenzlinii was one of the few species where seeds reached protocorm stages during in situ germination experiments. On the contrary, P. kraenzlinii grew on 21% of the R. inermis within our plots, but no embryo development was observed on this phorophyte species. Thus, population dynamics of orchids can be context dependent where best sites for one life history stage are not necessarily best for another stage. Indeed, we found that best sites for germination are not always the same as sites where plants can develop and survive, as observed by Crain et al. (2022) for epiphytic Lepanthes caritensis in the Carite State Forest in Puerto Rico, Whitman & Ackerman (2015) for terrestrial Prescottia stachyodes in El Yunque, Puerto Rico, and by Jacquemyn et al. (2007) for Orchis purpurea in Belgium (see also Gowland et al. 2011 and Jersáková & Malinová 2007). Moreover, Otero et al. (2007) found that the best sites for germination of P. monensis are different from those sites with high pollination, suggesting that the major production of seeds may occur far from suitable germination sites. Which is why, when developing conservation strategies for orchids, the environmental conditions in which an established population exists should not be assumed to be good for germination and establishment, unless recruitment is observed (Rasmussen et al. 2015). Another factor that needs to be considered is that of OMF usage throughout the life cycle of an orchid. Ontogenic turnover of OMF species exists in some orchids, suggesting that the OMF that trigger seed germination is not necessarily the best for later development (Otero et al. 2005, Bidartondo & Read 2008, Meng et al. 2019a, 2019b, Fernández et al. 2023).
Conclusion
Psychilis kraenzlinii was shown to prefer a subset of available phorophytes as well as higher probability of developing near established orchids. The orchid was found more often on substrates with a high WHC and lower FI (smoother bark). These results are not entirely consistent with similar studies of other orchids done under different climatic regimes and vegetation types, including closely related P. monensis, suggesting that preferences for certain substrate conditions may be context dependent. Psychilis kraenzlinii is the most widespread member of the genus, so comparative studies of different populations might reveal how environmental conditions affect phorophyte preferences. The results described here lay the foundation to develop informed conservation and management strategies for P. kraenzlinii and other species of the genus. However, various unknowns must be clarified: (1) pollinator identity and visitation frequency; (2) the OMF that triggers seed germination and development; (3) distribution of the orchid and how it relates to the distribution of its pollinators and OMF; (4) abiotic factors affecting the distribution of this orchid and its symbionts. Nonetheless, the population studied here is unusually large and apparently robust having evidence of fruit production and germination success which may be viewed generally as an indicator of a healthy population (Pierce & Belotti 2011). Still, this is a population near the edge of the Forest Reserve and should be monitored for any incursions and adjacent development which may affect critical ecosystem functions. The phorophyte that P. kraenzlinii prefers in the Susúa State Forest, M. portoricensis, is an endemic and rare shrub found in the southwest of Puerto Rico (Axelrod 2011). The protection and monitoring of this tree species might also be beneficial for P. kraenzlinii. It cannot be overstated, orchid conservation needs to target whole ecosystems, particularly in biodiversity hotspots of which the Caribbean is one (Fay 2018, Myers et al. 2000, Phillips et al. 2020).
Acknowledgements
NGO dearly thanks her grandmother, Maria M. Navarro Roldán, for making the plankton net seed packets used for her in situ seed germination experiment; Frank Axelrod and Steve Maldonado-Silvestrini for helping with phorophyte identification; Jamilys Rivera-Rodríguez, Elif Kardas, Carlos Cifuentes, Sergio L. Alicea Román, Armando Canchani, Angel M. Duran De Leon and Yalmarie Numan for their support during data collection and/or analysis; Dr. Paul Bayman, for making his lab available, we would have not been able to complete our experiments otherwise, for his comments and critiques along the way, and revision of the manuscript; to NGO thesis committee members, who shed the light to the correct path when she doubted; Puerto Rico Louis Stokes Alliance for Minority Participation Bridge to the Doctorate program (National Science Foundation HRD-1906130) and the American Orchid Society Conservation Committee for the financial support that made this research possible. Permits for this research were provided by the Department of Natural and Environmental Resources of Puerto Rico (Permit numbers: 2020-EPE-032 and 2021-EPE-041).
Literature cited
- Ackerman, J. D. (1986). Coping with the epiphytic existence: pollination strategies. Selbyana, 9(1), 52-60.
- Ackerman, J. D. & Collaborators. (2014). Orchid flora of the Greater Antilles Memoirs of the New York Botanical Garden, 105.
- Ackerman, J. D., Montalvo, A. M. & Vera, A. M. (1989). Epiphyte host specificity of Encyclia krugii, a Puerto Rican endemic orchid. Lindleyana, 4(2), 74-77.
-
Ackerman, J. D., Sabat, A. & Zimmerman, J. K. (1996). Seedling establishment in an epiphytic orchid: an experimental study of seed limitation. Oecologia, 106(2), 192-198. doi: 10.1007/BF00328598
» https://doi.org/10.1007/BF00328598 -
Adhikari, Y. P. & Fischer, A. (2011). Distribution pattern of the epiphytic orchid Rhynchostylis retusa under strong human influence in Kathmandu valley, Nepal. Botanica Orientalis: Journal of Plant Science, 8, 90-99. doi: 10.3126/botor.v8i0.5956
» https://doi.org/10.3126/botor.v8i0.5956 -
Aragón, S. & Ackerman, J. D. (2004). Does flower color variation matter in deception pollinated Psychilis monensis (Orchidaceae)? Oecologia, 138(3), 405-413. doi: 10.1007/s00442-003-1443-9
» https://doi.org/10.1007/s00442-003-1443-9 - Arditti, J. (1992). Fundamentals of orchid biology Wiley.
- Axelrod, F. S. (2011). A systematic vandemecum to the vascular plants of Puerto Rico Brit Press, 34.
-
Barthelme, S. (2023). imager: Image processing library based on ‘CImg’ (R package version 0.45.2). Retrieved from https://CRAN.R-project.org/package=imager
» https://CRAN.R-project.org/package=imager - Benzing, D. H. (1979). Alternative interpretations for the evidence that certain orchids and bromeliads act as shoot parasites. Selbyana 5(2), 135-144.
- Benzing, D. H. (1990). Vascular epiphytes. General biology and related biota Cambridge University Press.
- Benzing, D. H. (2004). Vascular epiphytes. In: D. M. Lowman & H. B. Rinker (2nd Ed.), Forest canopies (2nd Ed.) (pp. 175-211). Elsevier.
-
Bidartondo, M. I. (2005). The evolutionary ecology of myco-heterotrophy. New Phytologist, 167(2), 335-352. doi: 10.1111/j.1469-8137.2005.01429.x
» https://doi.org/10.1111/j.1469-8137.2005.01429.x -
Bidartondo, M. I. & Read, D. J. (2008). Fungal specificity bottlenecks during orchid germination and development. Molecular Ecology, 17(16), 3707-3716. doi: 10.1111/j.1365-294X.2008.03848.x
» https://doi.org/10.1111/j.1365-294X.2008.03848.x -
Brandner, S. (2005). An assessment of the fungal specificity of the orchid sub-tribe Pterostylis Centre for Australian National Biodiversity Research and Australian National Herbarium Retrieved from https://www.cpbr.gov.au/cpbr/summer-scholarship/2005-projects/brandner-susan-orchids/index.html
» https://www.cpbr.gov.au/cpbr/summer-scholarship/2005-projects/brandner-susan-orchids/index.html - Cabrera-García, B., Guerrero, A., Folgado, R., Jimenez, F. & Serra, C. (2023) Biología reproductiva de Psychilis truncata (Orchidaceae) en Arroyo Corral, Partido, Dajabón, República Dominicana. Moscosoa, 21, 99-114.
-
Callaway, R. M., Reinhart, K. O., Moore, G. W., Moore, D. J. & Pennings, S. C. (2002). Epiphyte host preferences and host traits: mechanisms for species-specific interactions. Oecologia, 132(2), 221-230. doi: 10.1007/s00442-002-0943-3
» https://doi.org/10.1007/s00442-002-0943-3 -
Crain, B. J., Sánchez-Cuervo, A. M. & Deren, V. (2022). Mixed evidence of a commensal relationship between a rare epiphytic orchid and cohabiting bryophytes. Botanical Journal of the Linnean Society, 201(4), pp. doi: https://doi.org/10.1093/botlinnean/boac049
» https://doi.org/https://doi.org/10.1093/botlinnean/boac049 -
Departamento de Recursos Naturales. (2015). El Bosque Estatal de Susua. Retrieved from http://drna.pr.gov/wp-content/uploads/2015/04/El-Bosque-Estatal-de-Susúa.pdf
» http://drna.pr.gov/wp-content/uploads/2015/04/El-Bosque-Estatal-de-Susúa.pdf -
Diez, J. M. (2007). Hierarchical patterns of symbiotic orchid germination linked to adult proximity and environmental gradients. Journal of Ecology, 95(1), 159-170.10.1111/j.1365-2745.2006.01194.x
» https://doi.org/10.1111/j.1365-2745.2006.01194.x -
Fay, M. F. (2018). Orchid conservation: how can we meet the challenges in the twenty-first century? Botanical Studies, 59(16), 1-6. doi: 10.1186/s40529-018-0232-z
» https://doi.org/10.1186/s40529-018-0232-z -
Fernández, M., Kaur, J. & Sharma, J. (2023). Co-occurring epiphytic orchids have specialized mycorrhizal fungal niches that are also linked to ontogeny. Mycorrhiza, 33(1-2), 1-19. doi: 10.1007/s00572-022-01099-w
» https://doi.org/10.1007/s00572-022-01099-w - Frei, S. J. K. (1973). Effect of bark substrate on germination and early growth of Encyclia tampensis seeds. American Orchid Society Bulletin, 42(8), 701-708.
-
Frei, S. J. K. & Dodson, C. H. (1972). The chemical effect of certain bark substrates on the germination and early growth of epiphytic orchids. Bulletin of the Torrey Botanical Club, 99(6), 301-307. doi: https://doi.org/10.2307/2997072
» https://doi.org/https://doi.org/10.2307/2997072 -
Gentry, A. H. & Dodson, C. H. (1987). Diversity and biogeography of neotropical vascular epiphytes. Annual Missouri Botanical Garden, 74(2), 205-233. doi: https://doi.org/10.2307/2399395
» https://doi.org/https://doi.org/10.2307/2399395 - González-Orellana, N., Salazar-Mendoza, A., Numan, Y. & Ackerman, J. D. (2022). Understanding orchid conservation, one species at a time. Orchids, 91(12), 914-919.
-
Gowland, K. M., Wood, J., Clements, M. A. & Nicotra, A. B. (2011). Significant phorophyte (substrate) bias is not explained by fitness benefits in three epiphytic orchid species. American Journal of Botany, 98(2), 197-206. Doi: 10.3732/ajb.1000241
» https://doi.org/10.3732/ajb.1000241 - Gravendeel, B., Smithson, A., Slik, F. J. W. & Schuiteman, A. (2004). Epiphytism and pollinator specialization: drivers for orchid diversity? Philosophical Transactions of the Royal Society London B, 359, 1523-1535.
-
Hietz, P. & Hietz-Seifert, U. (1995). Composition and ecology of vascular epiphyte communities along an altitudinal gradient in central Veracruz, Mexico. Journal of Vegetation Science, 6(4), 487-498. Doi: https://doi.org/10.2307/3236347
» https://doi.org/https://doi.org/10.2307/3236347 -
Izuddin, M., Srivathsan, A., Lee, A. L., Yam, T. W. & Webb, E. L. (2019a). Availability of orchid mycorrhizal fungi on roadside trees in a tropical urban landscape. Scientific Reports, 9(1), 1-12. Doi: 10.1038/s41598-019-56049-y
» https://doi.org/10.1038/s41598-019-56049-y -
Izuddin, M., Yam, T. W. & Webb, E. L. (2019b). Germination niches and seed persistence of tropical epiphytic orchids in an urban landscape. Journal of Plant Research, 132(3), 383-394. Doi: 10.1007/s10265-019-01110-0
» https://doi.org/10.1007/s10265-019-01110-0 -
Jacquemyn, H., Brys, R., Vandepitte, K., Honnay, O., Roldán-Ruiz, I. & Wiegand, T. (2007). A spatially explicit analysis of seedling recruitment in the terrestrial orchid Orchis purpurea New Phytologist, 176(2), 448-459. Doi: 10.1111/j.1469-8137.2007.02179.x
» https://doi.org/10.1111/j.1469-8137.2007.02179.x -
Jersáková, J. & Malinová, T. (2007). Spatial aspects of seed dispersal and seedling recruitment in orchids. New Phytologist, 176(2), 237-241. Doi: 10.1111/j.1469-8137.2007.02223.x
» https://doi.org/10.1111/j.1469-8137.2007.02223.x -
Khamchatra, N. M., Dixon, K., Chayamarit, K., Apisitwanich, S. & Tantiwiwat, S. (2016). Using in situ seed baiting technique to isolate and identify endophytic and mycorrhizal fungi from seeds of a threatened epiphytic orchid, Dendrobium friedericksianum Rchb.f. (Orchidaceae). Agriculture and Natural Resources, 50(1), 8-13. doi: https://doi.org/10.1016/j.anres.2016.01.002
» https://doi.org/https://doi.org/10.1016/j.anres.2016.01.002 -
Kartzinel, T. R., Trapnell, D. W. & Shefferson, R. P. (2013). Highly diverse and spatially heterogeneous mycorrhizal symbiosis in a rare epiphyte is unrelated to broad biogeographic or environmental features. Molecular Ecology, 22(23), 5949-5961. doi: 10.1111/mec.12536
» https://doi.org/10.1111/mec.12536 -
Laube, S. & Zotz, G. (2006). Neither host-specific nor random: vascular epiphytes on three tree species in a Panamanian lowland forest. Annals of Botany, 97(6), 1103-1114. doi: 10.1093/aob/mcl067
» https://doi.org/10.1093/aob/mcl067 -
McCormick, M. K., Taylor, D. L., Whigham, D. F. & Burnett Jr, R. K. (2016). Germination patterns in three terrestrial orchids relate to abundance of mycorrhizal fungi. Journal of Ecology, 104(3), 744-754. doi: https://doi.org/10.1111/1365-2745.12556
» https://doi.org/https://doi.org/10.1111/1365-2745.12556 -
Meng, Y. Y., Fan, X. L., Zhou, L. R., Shao, S. C., Liu, Q., Selosse, M. A. & Gao, J. Y. (2019a). Symbiotic fungi undergo a taxonomic and functional bottleneck during orchid seeds germination: a case study on Dendrobium moniliforme Symbiosis, 79, 205-212. doi: 10.1007/s13199-019-00647-x
» https://doi.org/10.1007/s13199-019-00647-x -
Meng, Y. Y., Zhang, W. L., Selosse, M. A. & Gao, J. Y. (2019b). Are fungi from adult orchid roots the best symbionts at germination? A case study. Mycorrhiza, 29(5), 541-547. doi: 10.1007/s00572-019-00907-0
» https://doi.org/10.1007/s00572-019-00907-0 -
Migenis, L. E. & Ackerman, J. D. (1993). Orchid-phorophyte relationships in a forest watershed in Puerto Rico. Journal of Tropical Ecology, 9(2), 231-240. doi: 10.1017/S0266467400007227
» https://doi.org/10.1017/S0266467400007227 -
Miller, J. S., Krupnick, G. A., Stevens, H., Porter-Morgan, H., Boom, B., Acevedo-Rodríguez, P., Ackerman, J. D., Kolterman, D., Santiago, E., Torres, C. & Velez, J. (2013). Toward Target 2 of the Global Strategy for Plant Conservation: An Expert Analysis of the Puerto Rican Flora to Validate New Streamlined Methods for Assessing Conservation Status. Annals of the Missouri Botanical Garden, 99(2), 199-205. doi: https://doi.org/10.3417/2011121
» https://doi.org/https://doi.org/10.3417/2011121 - Miller, G., & Lugo, A. E. (2009). Guide to the ecological systems of Puerto Rico USDA, IITF-GTR-35.
-
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853-858. doi: https://doi.org/10.1038/35002501
» https://doi.org/https://doi.org/10.1038/35002501 -
Otero, J. T., Ackerman, J. D. & Bayman, P. (2002). Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. American Journal of Botany, 89(11), 1852-1858. doi: 10.3732/ajb.89.11.1852
» https://doi.org/10.3732/ajb.89.11.1852 -
Otero, J. T., Aragón, S. & Ackerman, J. D. (2007). Site variation in spatial aggregation and phorophyte preference in Psychilis monensis (Orchidaceae). Biotropica, 39(2), 227-231. doi: 10.1111/j.1744-7429.2006.00258.x
» https://doi.org/10.1111/j.1744-7429.2006.00258.x -
Otero, J. T., Bayman, P. & Ackerman, J. D. (2005). Variation in mycorrhizal performance in the epiphytic orchid Tolumnia variegata in vitro: the potential for natural selection. Evolutionary Ecology, 19, 29-43. doi: 10.1007/s10682-004-5441-0
» https://doi.org/10.1007/s10682-004-5441-0 -
Otero, J. T. & Flanagan, N. S. (2006). Orchid diversity - beyond deception. Trends in Ecology and Evolution, 21(2), 64-65. doi: https://doi.org/10.1016/j.tree.2005.11.016
» https://doi.org/https://doi.org/10.1016/j.tree.2005.11.016 -
Petrolli, R., Augusto Vieira, C., Jakalski, M., Bocayuva, M. F., Vallé, C., Cruz, E. D. S., Selosse, M., Martos, F. & Kasuya, M. C. M. (2021). A fine-scale spatial analysis of fungal communities on tropical tree bark unveils the epiphytic rhizosphere in orchids. New Phytologist, 231(5), 2002-2014. doi: 10.1111/nph.17459
» https://doi.org/10.1111/nph.17459 -
Petrolli, R., Zinger, L., Perez-Lamarque, B., Collobert, G., Griveau, C., Pailler, T., Selosse, M. & Martos, F. (2022). Spatial turnover of fungi and partner choice shape mycorrhizal networks in epiphytic orchids. Journal of Ecology, 110(11), 2568-2584. doi: https://doi.org/10.1111/1365-2745.13986
» https://doi.org/https://doi.org/10.1111/1365-2745.13986 -
Petter, G., Wagner, K., Wanek, W., Sánchez Delgado, E. J., Zotz, G., Cabral, J. S. & Kreft, H. (2016). Functional leaf traits of vascular epiphytes: vertical trends within the forest, intra- and interspecific trait variability, and taxonomic signals. Functional Ecology, 30(2), 188-198. doi: https://doi.org/10.1111/1365-2435.12490
» https://doi.org/https://doi.org/10.1111/1365-2435.12490 -
Phillips, R. D., Reiter, N. & Peakall, R. (2020). Orchid conservation: from theory to practice. Annals of Botany, 126(3), 345-362. doi: https://doi.org/10.1093/aob/mcaa093
» https://doi.org/https://doi.org/10.1093/aob/mcaa093 - Pierce, S. & Belotti, J. (2011). The Conservation of Terrestrial Orchids from the Alps to the Po Plain Lombardy Parco delle Orobie Bergamasche and Centro Flora Autoctona della Regione Lombardia. Italy: Galbiate LC.
- Rasmussen, H. N. (1995). Terrestrial orchids: from seed to mycotrophic plant United Kingdom: Cambridge University Press.
-
Rasmussen, H. N., Dixon, K.W., Jersáková, J. & Těsǐtelová, T. (2015). Germination and seedling establishment in orchids: a complex of requirements. Annals of Botany, 116(3), 391-402. doi: 10.1093/aob/mcv087
» https://doi.org/10.1093/aob/mcv087 -
Rasmussen, H. N. & Rasmussen, F. N. (2018). The epiphytic habitat on a living host: reflections on the orchid-tree relationship. Botanical Journal of the Linnean Society, 186(4), 456-472. https://doi.org/10.1093/botlinnean/box085
» https://doi.org/https://doi.org/10.1093/botlinnean/box085 - Sanford, W. W. (1974). The Ecology of Orchids (pp. 1-100). In: C. L. Withner (1st Ed.), The orchids scientific studies
- Sauleda, R. P. (1988). A revision of the genus Psychilis Rafinesque (Orchidaceae). Phytologia, 65, 1-33.
-
Sáyago, R., Lopezaraiza-Mikel, M., Quesada, M., Álvarez-Añorve, M. Y., Cascante-Marín, A. & Bastida, J. M. (2013). Evaluating factors that predict the structure of a commensalistic epiphyte-phorophyte network. Proceedings of the Royal Society B, 280(1756), 20122821. doi: https://doi.org/10.1098/rspb.2012.2821
» https://doi.org/https://doi.org/10.1098/rspb.2012.2821 -
Siaz-Torres, S. S., Mora-Olivo, A., Arellano-Méndez, L. U., Vanoye-Eligio, V., Flores-Rivas, J. & de la Rosa-Manzano, E. (2021). Contribution of peeling host for epiphyte abundance in two tropical dry forests in the “El Cielo Biosphere Reserve”, Mexico. Plant Species Biology, 36(2), 269-283. doi: https://doi.org/10.1111/1442-1984.12317
» https://doi.org/https://doi.org/10.1111/1442-1984.12317 -
Stenberg, M. L. & Kane, M. E. (1998). In vitro seed germination and greenhouse cultivation of Encyclia boothiana var. erythronioides, an endangered Florida orchid. Lindleyana, 13(2), 101-112. doi: https://doi.org/10.1016/j.anres.2016.01.002
» https://doi.org/https://doi.org/10.1016/j.anres.2016.01.002 - Sulit, M. D. (1950). Field observations on tree hosts of orchids in the Philippines. Philippines Orchid Review, 3, 3-8.
- Sulit, M. D. (1953). Field observations on tree hosts of orchids in Palawan. Philippines Orchid Review, 5, 16.
- Szumilas, M. (2010). Explaining odds ratios. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 19(3), 227-229.
-
Tay, J. Y., Zotz, G. & Einzmann, H. J. (2023). Smoothing out the misconceptions of the role of bark roughness in vascular epiphyte attachment. New Phytologist, 238(3), 983-994. doi: https://doi.org/10.1111/nph.18811
» https://doi.org/https://doi.org/10.1111/nph.18811 -
Timsina, B., Rokaya, M. B., Münzbergová, Z., Kindlmann, P., Shrestha, B., Bhattarai, B. & Raskoti, B. B. (2016). Diversity, distribution, and host-species associations of epiphytic orchids in Nepal. Biodiversity and Conservation, 25(13), 2803-2819. doi: 10.1007/s10531-016-1205-8
» https://doi.org/10.1007/s10531-016-1205-8 -
Trapnell, D. W., & Hamrick, J. L. (2006). Variety of phorophyte species colonized by the neotropical epiphyte, Laelia rubescens (Orchidaceae). Selbyana, 27(1), 60-64. doi: 10.2307/41760261
» https://doi.org/10.2307/41760261 - Tremblay, R. L. (1997). Distribution and Dispersion Patterns of Individuals in Nine Species of Lepanthes (Orchidaceae). Biotropica, 29(1), 38-45.
-
Tremblay, R. L., Meléndez-Ackerman, E. J. & Kapan, D. (2006). Do epiphytic orchids behave as metapopulations? Evidence from colonization, extinction rates and asynchronous population dynamics. Biological Conservation, 129(1), 70-81. doi: 10.1016/j.biocon.2005.11.017
» https://doi.org/10.1016/j.biocon.2005.11.017 - Tremblay, R. L., Zimmerman, J. K., Lebrón, L., Bayman, P., Sastre, I., Axelrod, F. & Alers-García, J. (1998). Host specificity and low reproductive success in the rare endemic Puerto Rican orchid Lepanthes caritensis Biological Conservation, 85(3), 297-304.
-
Wagner, K., Mendieta-Leiva, G. & Zotz, G. (2015). Host specificity in vascular epiphytes: a review of methodology, empirical evidence and potential mechanisms. AoB plants, 7, plu092. doi: https://doi.org/10.1093/aobpla/plu092
» https://doi.org/https://doi.org/10.1093/aobpla/plu092 -
Whitman, M. & Ackerman, J. D. (2015). Terrestrial orchids in a tropical forest: best sites for abundance differ from those for reproduction. Ecology, 96(3), 693-704. doi: https://doi.org/10.1890/14-0104.1
» https://doi.org/https://doi.org/10.1890/14-0104.1 -
Zarate-García, A. M., Noguera-Savelli, E., Andrade-Canto, S. B., Zavaleta-Mancera, H. A., Gauthier, A. & Alatorre-Cobos, F. (2020). Bark water storage capacity influences epiphytic orchid preference for host trees. American Journal of Botany, 107(5), 1-9. doi: 10.1002/ajb2.1470
» https://doi.org/10.1002/ajb2.1470 -
Zi, X. M., Sheng, C. L., Goodale, U. M., Shao, S. C. & Gao, J. Y. (2014). In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza, 24(7), 487-499. doi: 10.1007/s00572-014-0565-8
» https://doi.org/10.1007/s00572-014-0565-8 -
Zotz, G., Hietz, P. & Einzmann, R. (2021). Functional ecology of vascular epiphytes. Annual Plant Reviews, 4(4), 869-906. doi: 10.1002/9781119312994.apr0777
» https://doi.org/10.1002/9781119312994.apr0777
Publication Dates
-
Date of issue
Jan-Apr 2024
History
-
Received
12 Oct 2023 -
Accepted
08 Apr 2024

 Host suitability for germination differs from that of later stages of development in a rare epiphytic orchid
Host suitability for germination differs from that of later stages of development in a rare epiphytic orchid

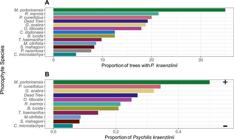



 B. Average number of P. kraenzlinii growing on the most common phorophyte species (Plus sign (+) marks a positive and significant association, whereas minus sign (-) marks a negative significant association.Associations based on residuals from Fisher Exact Test with Monte Carlo Simulation (p < 0.05, simulations = 2000).
B. Average number of P. kraenzlinii growing on the most common phorophyte species (Plus sign (+) marks a positive and significant association, whereas minus sign (-) marks a negative significant association.Associations based on residuals from Fisher Exact Test with Monte Carlo Simulation (p < 0.05, simulations = 2000).
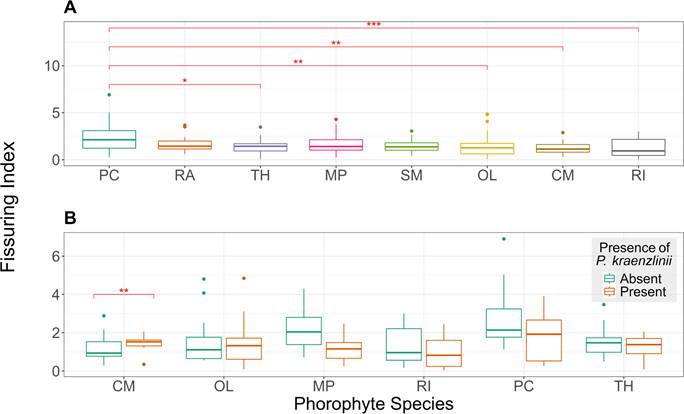 B. Box plots of the fissuring index of the bark of trees with and without Psychilis kraenzlinii among phorophyte species.Randia aculeata and Swietenia mahagoni not shown because data of trees with a P. kraenzlinii was not available.Red brackets with asterisks mark significant differences where: * = p ≤ 0.05; **= p ≤ 0.01; ***= p ≤ 0.001.
B. Box plots of the fissuring index of the bark of trees with and without Psychilis kraenzlinii among phorophyte species.Randia aculeata and Swietenia mahagoni not shown because data of trees with a P. kraenzlinii was not available.Red brackets with asterisks mark significant differences where: * = p ≤ 0.05; **= p ≤ 0.01; ***= p ≤ 0.001.
 Where Stage 1 refers to seeds with swollen embryos, Stage 2 are seeds whose embryo have swelled to the point of breaking the testa, Stage 3 the testa is gone and the protocorm is formed, and Stage 4 the protocorm has elongated.For Ouratea littoralis, no seeds developed near an established P. kraenzlinii, whereas in the case of Coccoloba microstachya and Randia aculeata, no packets were placed near and established orchid.Red lines indicate significant differences according to Conover-Iman Pairwise Comparisons (p<0.05).
Where Stage 1 refers to seeds with swollen embryos, Stage 2 are seeds whose embryo have swelled to the point of breaking the testa, Stage 3 the testa is gone and the protocorm is formed, and Stage 4 the protocorm has elongated.For Ouratea littoralis, no seeds developed near an established P. kraenzlinii, whereas in the case of Coccoloba microstachya and Randia aculeata, no packets were placed near and established orchid.Red lines indicate significant differences according to Conover-Iman Pairwise Comparisons (p<0.05).
 Photos by N. González-Orellana.A. Pictures of the bark surface of Coccoloba microstachya taken with a camera coupled to a dissecting microscope and a ring light. B. Pictures of the bark surface of Phyllanthus cuneifolius taken with a camera coupled to a dissecting microscope and a ring light. C. Photos converted to black and white (binary) images of C. microstachya. D. Photos converted to black and white (binary) images of P. cuneifolius. Enclosed in red is the area where roughness interpretation could be affected by bark pigmentation.
Photos by N. González-Orellana.A. Pictures of the bark surface of Coccoloba microstachya taken with a camera coupled to a dissecting microscope and a ring light. B. Pictures of the bark surface of Phyllanthus cuneifolius taken with a camera coupled to a dissecting microscope and a ring light. C. Photos converted to black and white (binary) images of C. microstachya. D. Photos converted to black and white (binary) images of P. cuneifolius. Enclosed in red is the area where roughness interpretation could be affected by bark pigmentation.