Abstract
During an expedition in which we randomly placed 341 plots across a large elevation range (1100- 3880 m) and spatial gradient (~270 km) in the Eastern Cordillera, we found a new species of the genus Lepanthes in Santuario de Fauna y Flora de Iguaque, Boyaca, Colombia. We propose L. bachue as a new species that is most similar to L. papallactae but it differs in its proliferous plants (vs. non proliferous), the petals with the upper lobe 2.5 mm long, the lower lobe 4.5 mm long, larger than the upper one, and the margins fimbriate (vs. lobes subequal, 4.5 mm long and shortly pubescent) and the lip with the blades oblong, sub-sigmoid, adnate to the middle of the column with a short oblong, bifid, an appendix hiding in the middle (vs. blades narrowly elliptical-oblong, adnate to the column above the middle with a recurved pedunculate, biglandular appendix). Our extensive sampling across different areas suggests that this species is geographically restricted and highly specialized in terms of habitat. Thus, it is urgent to protect its natural habitat and population.
Keywords: Eastern Cordillera; endemic; Lepanthes bachue; Neotropics; Protected área.
Resumen
En expediciones en la Cordillera Oriental, durante un muestreo aleatorio de 341 cuadrantes a lo largo de un amplio gradiente elevacional (1100-3880 m) y geográfico (~270 km), encontramos una especie nueva del género Lepanthes en el Santuario de Fauna y Flora de Iguaque, Boyacá, Colombia. Proponemos L. bachue como especie nueva similar a L. papallactae pero difiere por su crecimiento prolifero (vs. no prolifero), el lóbulo superior del labelo de 2.5 mm de largo, el lóbulo inferior 4.5 mm de largo, más largo que el lóbulo superior, y márgenes fimbriados (vs. lobulos de tamaño similar, 4.5 mm de largo cortamente pubescentes) y las láminas del labelo oblongas, sub-sigmoides, adnado a la mitad de la columna con un apéndice amarillo, corto, oblongo, bífido escondido en la mitad (vs. láminas estrechamente elíptico-oblongas, adnado a la mitad de la columna con un apéndice biglandular recurvado). Nuestro amplio muestreo, en términos de elevación y geográfico, evidencia que la nueva especie está geográficamente restringida al Santuario de Fauna y Flora de Iguaque presentando un alto nivel de especialización de hábitat, por tanto, es necesario proteger su hábitat natural y la población conocida hasta ahora.
Palabras clave: Área protegida; Cordillera Oriental; endémico; Lepanthes bachue; Neotrópico
Introduction
Orchidaceae Juss. is the most hyper diverse plant family in the Andes realm and the second richest family worldwide (Pérez-Escobar et al. 2022, WCSP 2019). This diversity has been protected by law in many countries, primarily attending the recommendation of the ''Convention on International Trade in Endangered Species of Wild Fauna and Flora'' (Ministerio de Ambiente, Vivienda y Desarrollo Territorial 2010). However, this biodiversity is under constant threat from biological resource use, agriculture, disturbance, and modifications to natural systems (Wraith & Pickering 2018). Recent estimates using an automated approach suggest that 31.2% of orchid species (4342 out of 13,910 evaluated species) may face extinction (Zizka et al. 2021). Local assessments based on IUCN criteria (IUCN 2020) indicate the alarming situation of orchids with unfinished work in threaten categorization and threats in Colombia (207 threatened species in Calderón-Saenz 2007), Peru (300 species, Roque & León 2006), and Ecuador (1421 species, Endara & Jost 2011). This high diversity continues expanding as orchid species are discovered every year because orchidologists are exploring new areas, and taxonomists, along with molecular biologists, are resolving taxonomical puzzles within this specious group (Pérez-Escobar et al. 2017).
Lepanthes Sw. (Swartz 1799; Pleurothallidinae) is one of the most species-rich neotropical genera with more than 1164 species with high levels of endemicity (Crain & Tremblay 2014, POWO 2022). This high biodiversity seems to respond to a rapid diversification process (Pérez-Escobar et al. 2017), despite being a recently evolved genus with a short divergence time (Bogarín et al. 2018). Although Lepanthes is comprised of many highly restricted species (Bogarín et al. 2018, Crain & Tremblay 2014), the genus also has some widespread species such as L. mucronata Lindl. (Lindley 1836, Moreno et al. 2020), and L. wageneri Rchb. (Luer & Thoerle 2012, Reichenbach 1855). This geographical restriction might be counterintuitive because Lepanthes seeds can disperse over long distances primarily through wind-dispersion. Yet, most Lepanthes species have limited geographic ranges and exhibit long-tailed dispersal kernels (Fernández et al 2003, Kindlmann et al. 2014, Mccall 2007, Tremblay 1997). Hence, the geographical restriction of Lepanthes might respond to dispersal limitation and species specific plant pollinator relationships on top of the speciation process (J. Moreno unpubl. data). Tremblay (1997) found that seeds can disperse within the immediate 4.8 meters from the mother plant. Furthermore, populations seem to set low number of fruits, with less than one successful migrant per generation and have low thermal tolerance to drought (Acevedo et al. 2020). Thus, many Lepanthes species seem not only to have small species ranges but also to be highly sensitive to disturbance.
Here, we describe a new species of Lepanthes of the eastern cordillera in the Colombian Andes. We randomly sampled 343 plots over 270 km in the eastern cordillera across a large elevational gradient (1100- 3880 m) and discovered the species dwelling in only one sampling plot in the Sanctuary of Fauna and Flora of Iguaque (hereafter SFFI) part of the National System of Protected Areas. Our sampling design shows the rarity of the new species even within the same area.
Materials and methods
Study area.- The sampling corresponds to the surveys carried out by the author EPS to understand the forces shaping orchid communities as part of the PARAMO project (Provisioning of ecosystem services and cultural values in the montane tropics). Here is a brief description of the study area and methodology, for further details please consult the author (unpubl. data). The study was in the departments of Boyacá, Cundinamarca, Meta, and Santander, in the east and west flanks of the Eastern Andes Cordillera in Colombia (Fig. 1). The study included three habitat types: 1forests, 2pasture for cattle, and 3paramo shrublands, grasslands, and forests of Polylepis Ruiz & Pav. All habitats have undergone historical human-mediated disturbance (landowners pers. comm.).
Sampling design.- The sampling was done between January 2018 and November 2021; we sampled 343 plots across 270 km distance covering a wide elevational range (1163-3763 m). We randomly placed 151 plots in Andean forests keeping at least a minimum of 172 m distance apart (range=172.3-2759 m), at a minimum of 30 m from the forest edge or roads. We placed 114 pasture plots, located at least 60 m away from the forest edge and at a minimum of 193 m apart (10 × 30 m each plot; range=193-4225 m). In paramos, we sampled natural shrublands (n=72 plots), along with high-elevation elfin forests (n=6 plots), located at a minimum of 195 m apart (range=195-15,170 m). Each sampling plot consisted of a 10 × 30 m quadrant. We sampled orchids in the understory up to 2 m above ground within each plot. Only adult individuals were recorded. Vouchers were preserved as dried or spirit specimens for future reference at VALLE and JAUM.
Descriptions and drawings.- Living and preserved specimens were examined for morphological and taxonomic comparisons. In addition, the monograph of Lepanthes from Colombia (Luer & Thoerle 2012) and other original descriptions from related species were reviewed and compared, specimens from the following herbaria: AMES, COL, CUVC, FMB, HUA, ICESI, JAUM, JBB, TOLI, and MO (online) were consulted. The description and drawings were prepared from living specimens dissected under a Barska AY11234 trinocular stereo microscope. Digital images were taken with a Canon 7d Mark II with a Canon 100 mm f/2.8L macro lens. Sketches from living and preserved specimens were digitized, and the images were used for diagramming a composite draft plate in Adobe Photoshop® 2020. In addition, a digital composite line drawing was made in the Procreate illustration application with an iPad 8th generation tablet. The Botanical terminology used in the manuscript followed Beentje (2010) and Stearn (1992).
Taxonomic treatment
Lepanthes bachue S.Vieira-Uribe, J.S.Moreno & E.Parra, sp. nov. (Fig. 2-4).
TYPE: Colombia. Boyacá: Municipio de Villa de Leyva, Santuario de Flora y Fauna Iguaque, 3160 m, 16 Feb 2021. Parra-Sánchez 2521 et al. (holotype: VALLE; isotype: JAUM-Spirit).
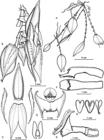
Lankester composite dissection plate of Lepanthes bachue S.Vieira-Uribe, J.S.Moreno & E.Parra.
Plants of Lepanthes bachue S.Vieira-Uribe, J.S.Moreno & E.Parra growing in their natural habitat.
Diagnosis: Lepanthes bachue is most similar to Lepanthes papallactae Luer & Hirtz (Luer 1987), but it can be distinguished by its proliferous plants (vs. non proliferous), the lateral sepals connate into an ovate-lanceolate synsepal (vs. ovate lateral sepals), the petals with the upper lobe 2.5 mm long, the lower lobe larger than the upper long, 4.5 mm long and the margins ciliate (vs. lobes subequal, 4.5 mm long and shortly pubescent) and the lip with the blades oblong, sub-sigmoid, adnate to the middle of the column with a short oblong, bifid appendix hiding in the middle (vs. blades narrowly elliptical-oblong, adnate to the column above the middle with a recurved, pedunculate, bi-glandular appendix).
Plant epiphytic, caespitose and proliferous, up to 29 cm tall. Roots slender, flexuous, filiform, ca. 0.6-1.5 mm in diameter. Ramicauls slender, suberect to pendent, 5.0-21.5 cm long, enclosed by 7-14 acuminate, ribbed, pilose sheaths with pilose dilated margins. Leaves coriaceous, acrodromous and reticulate-veined, ovate, the apex attenuated, 3.7-7.2 × 1.9-4.1 cm, the rounded base contracted into a 2.0-2.5 mm long petiole. Inflorescence a congested, distichous raceme successively many-flowered, up to 10 cm long including the peduncle, hanging below the surface of the leaf by a filiform, terete peduncle up to 5.5 cm long borne near the apex of the ramicaul; floral bracts conical, acuminate, sparsely ciliate, up to 1.8 mm long. Ovary terete, costate, ca. 2.4 mm long. Flowers with sepals claret to vermilion, petals and lip vermilion, the column vermilion with the apex claret, the anther cap magenta. Sepals lanceolate, concave, margins sparsely serrate, the veins abaxially carinate and covered with filiform papillae. Dorsal sepal acute, 3-veined, 7.3 × 2.2 mm, connate at the base to the lateral sepals for ca. 0.5 mm. Lateral sepals with an attenuate apex, 2-veined, 6.8 × 1.6 mm, connate at the base for ca. 3.0 mm into a concave, ovate-lanceolate synsepal. Petals transversally bilobed, puberulent, ciliate margins, 0.7 × 7.0 mm, the upper lobes oblong to narrowly triangular, acute, 2.5 mm long, the lower lobes narrowly triangular-linear, subfalcate, acute, 4.5 mm long. Lip bilaminate, the blades oblong, sub-sigmoid, the base obtuse, the apex obtuse, falcate, ciliate, 3.4 × 0.9 mm, supported by cuneate connectives from near the middle, the body orbicular, abaxially pubescent, the sinus with two rounded, pubescent protuberances with a short oblong, bifid, yellow appendix hiding in the middle; adnate to the middle of the column. Column cylindrical, 3.3 mm long, the anther and stigma apical. Pollinia 2, yellow, clavate, attached to a detachable viscidium, 1.1 mm long. Anther cap cucullate, obcordate. Capsule not seen.
Additional material studied: COLOMBIA. Boyacá: Villa de Leyva, Santuario de Fauna y Flora de Iguaque, 3000 m s.n.m. M Rincón-González et al 1971, 12/ IV/2021 (JBB 35407!).
Etymology: In reference to Bachué in native Chibcha language ''the one with the naked breast''. Bachué is the Muisca goddess of humanity. According to Muisca mythology, Bachué emerged of the waters in the Iguaque Lake and gave birth to humanity. The Iguaque Lake is a highland lake (3570 m), an important cultural, spiritual, and social landmark of the protected area where the species was found.
Taxonomic notes: The most similar species to Lepanthes bachue is undoubtedly L. papallactae (Hirtz 1810, MO; Fig. 5), endemic to Ecuador, with dull redbrown sepals with yellow margins and purple-brown petals (vs. claret and vermillion) and with some morphological differences as discussed in the diagnosis. Also, from Colombia and Ecuador, Lepanthes aries Luer (Luer 1983), resembles the new species but can be distinguished by its non-proliferous habit (vs. prolific habit), oblong leaves (vs. ovate), the flowers with the lateral sepals elliptic-lanceolate (vs. lanceolate), the petals with the upper lobe elliptical and the lower lobe triangular (vs. the upper lobe oblong to narrowly triangular and the lower lobes narrowly triangular-linear); the lip appendix 3-lobed with one lobe beneath the other 2 lobes in the sinus (vs. bifid, hiding in the middle of the sinus) and the column 2.5 mm long (vs. 3.3 mm long).
Habitat and ecology: The new species is known only from the type locality (Fig. 1, 6) where it grows in groups in shaded areas hanging from tree trunks and branches (Fig. 4). We received a report of a plant photographed by Luis Pina growing in the vicinity of Arcabuco (<10 km distance from SFFI). However, we could not verify the material and the origin of the plant, then it is uncertain whether a population exists in the wild of the photographed plant. Plants of the new species are unfrequently found in a single place at 3150 m where it grows as an epiphytic plant on lianas and fallen trees. The species has been found flowering between April and November, although the park rangers have observed it blooming almost all year round.
Floral comparison of Lepanthes bachue S.Vieira-Uribe, J.S.Moreno & E.Parra and L. papallactae Luer & Hirtz.
View of the forests in the Sanctuary of Fauna and Flora of Iguaque where Lepanthes bachue grows.
Conservation status: Lepanthes bachue is known only from its type locality, located in a government-managed protected area. Although the proposed IUCN categorization for this species is data deficient (DD), we provide an overview that can be use as population and distribution information to assess the species.
The species was found growing in large numbers (over 180 adult individuals) in the understory (tree density= 0.16 trees per m2) in only one site out of 343 sampling plots (Fig. 3). Our sampling design allowed us to randomly survey 12 plots (30 × 10 m each plot) within the protected area as well as other forests and pastures nearby (31 plots within 20 km radius), along the 2640 m elevational gradient (1120-3760 m). Furthermore, the staff of the SFFI has been continuously tracking the species for over a year across different parts of the protected area and have not seen it elsewhere. It is expected that the population or the habitat will not decline due to the current protection status (criteria= B-D), however the species has a very restricted geographic range limited to SFFI.
Lepanthes bachue is a rare species that has a narrow geographic distribution, specialized habitat requirements, and a small regional population. It might be facing great danger because rare species are more likely to go extinct than common species (Enquist et al. 2019, Pimm et al. 2014). Furthermore, unassessed species and DD species are less likely to receive funding for conservation by government and conservation institutions due to the uncertainty of their extinction risk (Bland et al. 2017, Sousa-Baena et al. 2014). Thus, we suggest the specie must be considered as of conservation concern and actions should be directed to protect its natural habitat and implement propagation in situ to improve the chances of survival, as well as to explore other locations within the Sanctuary to extend the potential habitats where the species may dwell.
Acknowledgements.
We would like to express our gratitude to the community of the vicinity of the Santuario who met with us to discuss the specific epithet of the species' name and was an essential part of the PARAMO project. Also, we are grateful to the staff of Santuario de Fauna y Flora de Iguaque who enthusiastically carried out further fieldwork to determine the spatial extent of the new entity. This is publication #44 of the Biodiversity, Agriculture, and Conservation in Colombia (Biodiversidad, Agricultura, y Conservación en Colombia (BACC)) project. Fieldwork in the forests was carried out on private properties with each landowner's permission. Plants were collected under the joint permit between Instituto Alexander von Humboldt and PARAMO number 20192300064121.
Author contributions. SVU, SM and EPS conceived the idea; SVU and SM elaborated the taxonomic treatment of the species; EPS designed the sampling methodology and lead the ecological angle of the paper; SVU designed the Lankester Composite Dissection Plate and line drawing with photographs taken by SVU. All the authors contributed critically to the drafts and gave final approval for publication.
Literature cited
-
Acevedo, M. A., Beaudrot, L., Meléndez-Ackerman, E. J., & Tremblay, R. L. (2020). Local extinction risk under climate change in a neotropical asymmetrically dispersed epiphyte. Journal of Ecology, 108(4), 1553-1564. doi:10.1111/13652745.13361
» https://doi.org/10.1111/13652745.13361 - Beentje, H. (2010). The Kew Plant Glossary an illustrated dictionary of plant Terms Royal Botanical Gardens, Kew (eds). Richmond, UK: Kew Publishing.
-
Bland, L. M., Bielby, J., Kearney, S., Orme, C. D. L., Watson, J. E. M., & Collen, B. (2017). Toward reassessing datadeficient species. Conservation Biology, 31(3), 531-539. doi:10.1111/cobi.12850
» https://doi.org/10.1111/cobi.12850 -
Bogarín, D., Pérez-Escobar, O. A., Groenenberg, D., Holland, S. D., Karremans, A. P., Lemmon, E. M., … Gravendeel, B. (2018). Anchored hybrid enrichment generated nuclear, plastid and mitochondrial markers resolve the Lepanthes horrida (Orchidaceae: Pleurothallidinae) species complex. Molecular Phylogenetics and Evolution, 129, 27-47. doi:10.1016/j.ympev.2018.07.014
» https://doi.org/10.1016/j.ympev.2018.07.014 - Calderon-Saenz, E. (2007). Libro Rojo de Plantas de Colombia. Volúmen 6: Orquídeas, Primera Parte (E. CALDERON-SAENZ, Ed.) (Serie de L). Bogotá: Instituto Alexander von Humboldt Ministerio de Ambiente, Vivienda y Desarrollo Territorio.
-
Crain, B. J., & Tremblay, R. L. (2014). Do richness and rarity hotspots really matter for orchid conservation in light of anticipated habitat loss? Diversity and Distributions, 20(6), 652-662. doi:10.1111/ddi.12179
» https://doi.org/10.1111/ddi.12179 - Endara, L., & Jost, L. (2011). Lista roja de Orchidaceae. In S. León-Yánez, R. Valencia, N. Pitman, L. Endara, C. Ulloa, & H. Navarrete (Eds.), Libro rojo de las plantas endemicas del Ecuador (Second). Quito, Ecuador: Pontificia Universidad Católica del Ecuador.
-
Enquist, B. J., Feng, X., Boyle, B., Maitner, B., Newman, E. A., Jørgensen, P. M., … McGill, B. J. (2019). The commonness of rarity: Global and future distribution of rarity across land plants. Science Advances, 5(11), 1-14. doi:10.1126/ sciadv.aaz0414
» https://doi.org/10.1126/ sciadv.aaz0414 - Fernández, D. S., Tremblay, R. L., Ackerman, J. D., Rodríguez, E., & López, L. N. (2003). Reproductive potential, growth rate and light environment in Lepanthes rupestris Stimson. Lankesteriana, 7, 73-76.
-
Hansen, M. C., Potapov, P. v., Moore, R., Hancher, M., Turubanova, S. A., Tyukavina, A., … Townshend, J. R. G. (2013). High-Resolution Global Maps of 21st-Century Forest Cover Change. Science, 342(6160), 850-853. doi:10.1126/science.1244693
» https://doi.org/10.1126/science.1244693 - IUCN. (2020). The IUCN Red List of threatened species v. 2019-3. Gland, Switzerland.
-
Kindlmann, P., Meléndez-Ackerman, E. J., & Tremblay, R. L. (2014). Disobedient epiphytes: colonization and extinction rates in a metapopulation of Lepanthes rupestris (Orchidaceae) contradict theoretical predictions based on patch connectivity. Botanical Journal of the Linnean Society, 175(4), 598-606. doi:10.1111/boj.12180
» https://doi.org/10.1111/boj.12180 - Lindley, J. (1836). Notes of some genera and species of American Orchidaceae. Companion to the Botanical Magazine 2: 356.
- Luer, C. (1983). New species of Lepanthes (Orchidaceae). Phytologia 54(5): 329.
- Luer, C. A. (1987). New species from Ecuador. Lindleyana 2(2): 102.
- Luer, C. A., & Thoerle, L. (2012). Icones Pleurothallidinarum XXXII. Lepanthes of Colombia (Orchidaceae) (123rd ed.). Missouri, USA: Monographs in Systematic Botany from the Missouri Botanical Garden.
-
Mccall, K. (2007). Precipitation variation and its effects on the reproductive success and survival of Lepanthes jimenezii (Orchidaceae). University of South Florida, Florida, US. Retrieved from https://digitalcommons.usf.edu/tropical_ecology/547
» https://digitalcommons.usf.edu/tropical_ecology/547 - Ministerio de Ambiente, Vivienda y Desarrollo Territorial (2010). Resolución Número (956) 19 de mayo de 2010. ''Por la cual se declara en Colombia el 2010 como Año Nacional de las Orquídeas''. Bogota.
-
Moreno, J. S., Sandoval-Arango, S., Palacio, R. D., Alzate, N. F., Rincón, M., Gil, K., … Hazzi, N. A. (2020). Distribution Models and Spatial Analyses Provide Robust Assessments of Conservation Status of Orchid Species in Colombia: The Case of Lephantes mucronata Harvard Papers in Botany, 25(1), 111-121. doi:10.3100/hpib.v25iss1.2020.n14
» https://doi.org/10.3100/hpib.v25iss1.2020.n14 -
Pérez-Escobar, O. A., Chomicki, G., Condamine, F. L., Karremans, A. P., Bogarín, D., Matzke, N. J., … Antonelli, A. (2017). Recent origin and rapid speciation of Neotropical orchids in the world's richest plant biodiversity hotspot. New Phytologist, 215(2), 891-905. doi:10.1111/nph.14629
» https://doi.org/10.1111/nph.14629 -
Pérez-Escobar, O. A., Zizka, A., Bermúdez, M. A., Meseguer, A. S., Condamine, F. L., Hoorn, C., … Chomicki, G. (2022). The Andes through time: evolution and distribution of Andean floras. Trends in Plant Science Elsevier Ltd. doi:10.1016/j.tplants.2021.09.010
» https://doi.org/10.1016/j.tplants.2021.09.010 -
Pimm, S. L., Jenkins, C. N., Abell, R., Brooks, T. M., Gittleman, J. L., Joppa, L. N., … Sexton, J. O. (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science, 344(6187), 12467521-124675210. doi:10.1126/science.1246752
» https://doi.org/10.1126/science.1246752 - POWO. (2022). The International Plant Names Index and World Checklist of Vascular Plants.
- Reichenbach, H. G. (1855). Wagener's Orchideen aus Ocana. Bonplandia 3: 70.
-
Roque, J., & León, B. (2006). Orchidaceae endémicas del Perú. Revista Peruana de Biologia, 13(2), 759-878. Retrieved from http://sisbib.unmsm.edu.pe/BVRevistas/biologia/biologiaNEW.htm
» http://sisbib.unmsm.edu.pe/BVRevistas/biologia/biologiaNEW.htm - Stearn, W.T. (1992). Botanical Latin (4th ed.). Portland, USA: Timber Press.
- Swartz, O. (1799) Lepanthes Nova Acta Regiae Societatis Scientiarum Upsaliensis 6: 85.
-
Sousa-Baena, M. S., Garcia, L. C., & Townsend Peterson, A. (2014). Knowledge behind conservation status decisions: Data basis for ''Data Deficient'' Brazilian plant species. Biological Conservation, 173, 80-89. doi:10.1016/j.biocon.2013.06.034
» https://doi.org/10.1016/j.biocon.2013.06.034 -
Tadono, T., Ishida, H., Oda, F., Naito, S., Minakawa, K., & Iwamoto, H. (2014). Precise Global DEM Generation by ALOS PRISM. ISPRS Annals of the Photogrammetry, Remote Sensing and Spatial Information Sciences, II-4, 71-76. doi:10.5194/isprsannals-II-4-71-2014
» https://doi.org/10.5194/isprsannals-II-4-71-2014 -
Tremblay, R. L. (1997). Distribution and dispersion patterns of individuals in nine species of Lepanthes (Orchidaceae). Biotropica, 29(1), 38-45. doi:10.1111/j.1744-7429.1997.tb00004.x
» https://doi.org/10.1111/j.1744-7429.1997.tb00004.x - WCSP. (2019). World Checklist of Selected Plant Families .
-
Wraith, J., & Pickering, C. (2018). Quantifying anthropogenic threats to orchids using the IUCN Red List. Ambio, 47(3), 307-317. doi:10.1007/s13280-017-0964-0
» https://doi.org/10.1007/s13280-017-0964-0 -
Zizka, A., Silvestro, D., Vitt, P., & Knight, T. M. (2021). Automated conservation assessment of the orchid family with deep learning. Conservation Biology, 35(3), 897-908. doi:10.1111/cobi.13616
» https://doi.org/10.1111/cobi.13616
Data availability
Data citations
POWO. (2022). The International Plant Names Index and World Checklist of Vascular Plants.
WCSP. (2019). World Checklist of Selected Plant Families .
Publication Dates
-
Date of issue
May-Aug 2023
History
-
Received
16 Feb 2023 -
Accepted
24 Apr 2023

 A new species of Lepanthes (Pleurothallidinae) in honour of ''Bachué'', the mythological mother of the indigenous Muisca people
A new species of Lepanthes (Pleurothallidinae) in honour of ''Bachué'', the mythological mother of the indigenous Muisca people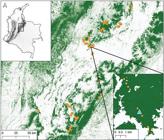





 Map insets show the elevation across Colombia where dark colours depict higher elevations (top left, ALOS 30-m-resolution digital elevation model;
Map insets show the elevation across Colombia where dark colours depict higher elevations (top left, ALOS 30-m-resolution digital elevation model;  A. Habit. B. Flower, oblique view. C. Dissected perianth. D. Lip, expanded and close-up to the bifid appendix. E. Ovary, column, and lip, lateral view. F. Column, lateral and dorsal views. G. Anther cap and pollinarium. Drawn by S.Vieira-Uribe from the plant that served as type (Parra-Sanchez 2521, VALLE).
A. Habit. B. Flower, oblique view. C. Dissected perianth. D. Lip, expanded and close-up to the bifid appendix. E. Ovary, column, and lip, lateral view. F. Column, lateral and dorsal views. G. Anther cap and pollinarium. Drawn by S.Vieira-Uribe from the plant that served as type (Parra-Sanchez 2521, VALLE).
 A. Habit. B. Flower, oblique view. C. Dissected perianth. D. Lip, expanded. E. Ovary, column, and lip, lateral view. F. Column, lateral and dorsal views. G. Anther cap and pollinarium. Photographed and assembled by S.Vieira-Uribe from the plant that served as type (Parra-Sanchez 2521, VALLE).
A. Habit. B. Flower, oblique view. C. Dissected perianth. D. Lip, expanded. E. Ovary, column, and lip, lateral view. F. Column, lateral and dorsal views. G. Anther cap and pollinarium. Photographed and assembled by S.Vieira-Uribe from the plant that served as type (Parra-Sanchez 2521, VALLE).

 Photography of Lepanthes bachue taken by S.Vieira-Uribe and L. papallactae taken by Andreas Kay under permission of Lou Jost.
Photography of Lepanthes bachue taken by S.Vieira-Uribe and L. papallactae taken by Andreas Kay under permission of Lou Jost.
 Photography by S.Vieira-Uribe.
Photography by S.Vieira-Uribe.