Abstract.
We propose Lepanthes chalalensis as a newly identified species confined to the north-east Andes of Colombia. We randomly placed 341 sampling plots across the Eastern Cordillera, including transformed and natural habitats. Here, we provide a detailed description, illustrating images, ecological discussions, a taxonomic key for the new entity, and a conservation status analysis. The species is highly geographically restricted (in 2 out of 341 sampling plots) and has a low population size (26 adult individuals). While L. chalalensis shows a resemblance to L. velosa from Ecuador, it can be distinguished by the wide lower lobe of the petals, which is long ciliated, the lip laminae, which are reduced and bear stiff cilia, and the appendix length, which appear to be twice as long as the lip blades. The species should be considered a conservation concern due to its high rarity.
Keywords: Eastern Cordillera; endemics; Neotropics; standardized sampling
Resumen.
Proponemos Lepanthes chalalensis como una nueva especie confinada al noreste de los Andes de Colombia. La nueva especie fue hallada en un muestreo de 341 parcelas aleatoriamente posicionadas a lo largo de la Cordillera Oriental, incluyendo hábitats transformados y naturales. Se proporcionan la descripción detallada, imágenes ilustrativas, discusiones ecológicas y un análisis del estado de conservación. La especie está altamente restringida geográficamente (en 2 de las 341 parcelas de muestreo) y tiene un tamaño de población bajo (26 individuos adultos). Aunque L. chalalensis muestra una similitud con L. velosa de Ecuador, puede distinguirse por el lóbulo inferior de los pétalos, que es ancho y presenta una cilios largos, las láminas del labelo, que están reducidas y presentan cilios rígidos, y la longitud del apéndice, que parece ser el doble de la longitud de las láminas del labelo. La especie debe considerarse una preocupación de conservación debido a su alta rareza.
Palabras clave: Cordillera Oriental; endémico; muestreo estandarizado; Neotrópico
Introduction
The environmental crisis caused by anthropogenic activities is driving species to extinction at rates never seen before (Ceballos et al. 2015). Threats such as habitat loss, fragmentation, and climate change are the main drivers of this negative trend across biological groups (Travis 2003). However, these drivers do not impact all species in the same way. For instance, 77.3% of new plant species to science are considered rare species and more likely to face extinction than wide-spread species (Brown et al. 2023). Rare species are those species with a restricted geographical range, low population size, and high habitat specialization (Rabinowitz 1981). Among plant families, Orchidaceae Juss. (Magnoliopsida) hold high levels of rarity, with an estimated 40% of species threatened (Zizka et al. 2021) and the highest proportion of endangered species among all plant families by trading (Hinsley et al. 2018). New orchid species are discovered every year in small populations and small ranges where natural habitats are highly transformed (Haddad et al. 2015, Parra-Sánchez 2023b, 2023c).
Lepanthes Sw. (Pleurothallidinae) is a neotropical genus of the Orchidaceae with 1196 species (Karremans et al. 2023). The genus is characterized by ramicauls covered with infundibuliform sheaths, often referred to as “lepanthiform sheaths,” each bearing a single leaf that supports a slender, elongated inflorescence carrying one or multiple flowers. These flowers typically feature transversely lobed petals, and, in most cases, a specialized structure known as an appendix on the lip, facilitating pollination through pseudocopulation (Blanco & Barboza 2005, Luer 1996). Although the genus has many geographically restricted species (Crain & Tremblay 2014), there are some widespread species (e.g. Lepanthes mucronata Lindl., Moreno et al. 2020). Crain & Tremblay (2014) found that 70% of their Lepanthes records occurred in less than three localities (793 out of 1126 species). Drivers of rarity in Lepanthes include rapid diversification processes with short divergence time (5-10 Mya; Bogarín et al. 2018, Pérez-Escobar et al. 2017), dispersal limitation (Acevedo et al. 2020, Kindlmann et al. 2014, Tremblay 1997), highly specific pollination relationships (Blanco & Barboza 2005), and habitat specificity (Luer & Thoerle 2012). These idiosyncratic features might explain why 73% of the 564 Lepanthes species globally assessed are threatened with extinction (BGCI 2023).
We discovered a new species of Lepanthes belonging to the section Lepanthes, and morphologically similar to Lepanthes deformisLuer & Hirtz (1987) and Lepanthes velosa Luer & Hirtz (Karremans et al. 2021). These species are all characterized by a column twisted about 45 degrees and bending to one side. The discovery was made in the community of Virolin, Santander, in the eastern cordillera of the Colombian Andes. For the new species, we provide a description, diagnostic images, a discussion with the related affinities, and a discussion of its conservation status.
Materials and methods
Study area.- We sampled natural and transformed habitats in a randomised design in the Departments of Cundinamarca, Boyacá, Meta, and Santander in Colombia. Following Parra-Sánchez et al. (2023a), sampling points covered natural habitats and pastures across a 2252 m elevational range (1163-3415 m; Tadono et al. 2014) and a 2937 mm precipitation range (879-3817 mm per year).
Sampling design.- Sampling plots comprised Andean and Altoandino forests (Etter et al. 2021) with an average cloud cover of 82% (Wilson & Jetz 2016). In total, we sampled 206 natural habitat plots (148 forest, 48 paramo, and 10 paramo forest), and 135 transformed habitat plots (90 Andean transformed and 45 paramo transformed, Vancutsem et al. 2021; Fig. 1). We sampled all understorey orchid individuals at each plot of 10 × 30 m from the ground floor up to 2 m. Identification of species or morphospecies was conducted following specialized literature and consultancy with local experts at the Herbarium VALLE.
Distribution of related species of Lepanthes chalalensis E.Restrepo & E.Parra, and study area in the Eastern Cordillera of the Colombian Andes.
Descriptions and illustrating material.- We found a species morphologically similar to Lepanthes velosa and L. deformis that we propose as a new species to science. The flowers of the new entity were dissected, and characters were measured to prepare the description and protologue. Vegetative structures and reproductive structures were measured from living and spirit material. The botanical terminology used for the description followed Stearn (1992) and Luer & Thoerle (2012). We revised the original descriptions of the sister species and the most recent monographs of the genus, for Colombia (Luer & Thorle 2012), Ecuador (Luer 1996), and Venezuela (Romero-González & Carnevali 2000). Nine herbaria were consulted (AMES, CAUP, COL, CUVC, HUA, JAUM, JBB, VALLE, and MO).
Finally, illustrations and taxonomic revisions for each of the Lepanthes species to which the new entity was compared. Illustration done with Procreate® version 5.3.7. Figures and analyses were prepared using specialized software for composite plate, Adobe Photoshop® 2019, and maps on QGis 3.16 (QGIS 2021).
Taxonomic treatment
Lepanthes chalalensis E.Restrepo & E.Parra, sp. nov. (Fig. 2 - 4).
TYPE: Colombia. Santander: Municipio de Virolín, Hacienda la Argentina, 2880 m a.s.l., February 2019, E. Parra-Sánchez 2401 (holotype: VALLE).
Diagnosis: Lepanthes chalalensis resembles the Ecuadorian Lepanthes velosa but can be easily distinguished by several characteristics. The lower lobe of the petals in L. chalalensis is much wider, measuring 0.93 mm, oblong, with a rounded apex and long cilia. Furthermore, the combination of the dimensions of the body, sinus and appendix in L. chalalensis is longer, about 1.62 mm, nearly twice as long as the lip blades when placed in their natural position, including the veil, whereas in L. velosa, the combined dimension of thebody, sinus and appendix is about 1.2 mm long, nearly as long as the lip, approximately.
Plant, epiphytic, caespitose up to 8.5 cm tall; roots slender. Ramicauls slender, horizontal to pendant, 5.00-5.54 cm long, enclosed by 6-7 blackish, tightly fitting, scabrous lepanthiform sheaths. Leaves erect, coriaceous, ovate-elliptical, 4.02-4.56 × 2.29-3.32 cm, the base broadly cuneate, contracted into a petiole 4.81 mm long, the apex shortly acuminate, emarginate with the mid vein extending beneath and ending in a short mucro. Inflorescence a very dense, distichous, successively flowered raceme up to 1.41-2.28 cm long, borne at the abaxial side of the leaf by a filiform peduncle 1.61-2.32 cm long; floral bracts slender, ca. 1 mm long, sparsely spiculate; pedicel 4.67-5.84 mm long; ovary terete, glabrous, keeled, 1.91-2.5 mm long; anatomically, all flower parts contain silver-like crystals, sepals hyaline, light yellow, glabrous, carinate externally, dorsal sepal ovate, acute, 3.47-4.90 × 2.66-2.80 mm, 3-veined, connate to the lateral sepals for ca. 1.22 mm; lateral sepals connate into a bifid lamina, , each being ovate-oblong, the apex attenuate, 2-veined, 3.78-4.44 × 1.22-2.12 mm connate for 1.73-2.00 mm; petals yellow suffused with red at the margins, transversely bilobed, microscopically pubescent, 1.33-1.54 × 3.31-3.58 mm wide, 1-veined, the upper lobe oblong with rounded end, ca. 1.54 wide, the lower lobe identical to the upper one, long ciliate, ca. 1.33 mm wide; lip red, bilaminate, covering two thirds of the column, the blades subrectangular, thickly verrucose, 0.45 × 0.27 each, cellular-papillose at the base, the anterior margins with a row of long, stiff cilia, connated beyond the base into a membranous, veil-like lamina; the body broad, the connectives narrowly oblong, born at either side of the lip; the body and sinus occupied by an oblong, minute appendix, the combined dimensions of the last 1.62 mm long, shallowly 3-veined, pubescent, with an ovoid, minutely pubescent bifid gland at the tip; column terete, 1.04-1.20 mm long, bent to one side, the anther cap deciduous, pollinia not seen fruit not seen.
Etymology: In reference to “Chalala” the territory of the “Guane” indigenous tribe settled in the territory currently known as Charalá in Santander, Colombia. The translation of “Chalala” from the Guane dialect is subject to dispute among anthropologists and historians. However, historical records trace back the name to the Indigenous Cacique Chalala, the leader of the tribe. The specific epithet of the species was selected by the community where the species was found.
Taxonomic discussion: Lepanthes chalalensis belongs to the section Lepanthes subsection Lepanthes. The new species exhibits an unusual development of the lip structures, similar to that seen in L. deformis and L. velosa. In these species, the lip blades are united into a thin, irregularly veined veil that overlys the column, which is twisted about 45 degrees and bent to the left (Luer & Thoerle 2012; Fig. 4B-C, Fig. 5). Lepanthes chalalensis can be distinguished for a series of floral morphological differences from similar species and elevations that species dwell in. The new species grows at ~2800 m, while L. deformis and L velosa are found relatively in the lowlands, at 750-1100 m. The closer species to L. chalalensis is L. velosa, which can be distinguished by a set of morphological differences in the lower lobe of the petals, which is much wider, oblong, with rounded apex, long ciliated ca. > 1 mm long (vs. narrowly triangular, short ciliate; ca. < 1 mm long). The key differences in the lip structures between L. chalalensis and L. velosa are the somewhat subrectangular and strongly reduced, more or less equal in L. chalalensis, with the anterior margins forming a row of long stiff cilia, and a much longer, oblong, 3-veined sinus (vs. also reduced, somewhat bigger blades, presenting a membranous, longitudinally microscopically veil-like lamina, and a smaller, shallowly channelled (unveined) appendix; Fig. 5). Furthermore, the relationship between the length in natural position of the sinus and the appendix, which L. chalalensis appears to be twice as long as the lip blades (vs. as long as the lip, ca. 1.2 mm long).
The other morphologically similar species is Lepanthes deformis, which has been found in the western cordillera of Colombia (vs. eastern cordillera of the new taxon). The new entity can be easily distinguished from L. deformis by the lateral sepals, which are nearly as wide as the dorsal sepal (vs. twice as wide as the dorsal sepal; both ca. 4 mm wide), the petals, which have subequal lobes, the lower oblong and long ciliated (vs. unequal, the lower much narrower, short ciliated (<1mm long). Finally, the appendix of the new species is longer, ca. 1.42 mm long, about twice the length of the lip blades (vs. appendix minute, not protruding, the lip laminae about 1.2 cm long). Likewise, the blade length in L. deformis is larger compared to Lepanthes chalalensis, and the sinus is somewhat reduced, ovoid, with a minute and ciliated appendix at the apical part, placing morphologically distant the grade of similarity of this species in the scheme (Fig. 5). Lepanthes chalalensis has “silver-like” crystals, that we speculate could be calcium oxalate (Chase & Peacor 1987). This feature is shared by at least one more species with 45 degrees twisted column not threated here (Pérez-Escobar pers. comm.). The actual role of these crystals is uncertain, but these crystals have been proposed to act as pseudonectar clusters for attracting pollinators (Chase & Peacor 1987), or crystal deposits (Luer 1990).
Habitat and ecology: The species is exclusively known from its type locality (Fig. 1). Our sampling indicates that it is infrequently encountered at 2200 m in elevation, where it grows epiphytically on lianas and fallen trees. Flowering has been observed throughout the year.
Conservation status: According to the IUCN criteria, this species might qualify for the Data Deficient (DD) category (IUCN 2020). However, our data suggests that it warrants conservation concern. We conducted random sampling across a 270 km south-to-north gradient and a 2640 meters elevational gradient (1120- 3760 m; Fig. 1). In 341 plots (10 m × 30 m), we found only 26 adult individuals growing epiphytically in the understory in only two plots (tree density = 0.21 - 0.26 trees per m2). Specifically, nine plots were within the same forest, and 24 plots were within a 10 km radius (Fig. 1). Our records indicate that the species appears to be geographically rare, with a small population size and specialized habitat requirements.
Population dynamics studies reveal occurrences of low fruit sets (Blanco & Barboza 2005, Tremblay et al. 1998), and evidence suggests that small plants experience the highest mortality in their natural habitat (Acevedo et al. 2020). Additionally, the sister species Lepanthes deformis is traded in the orchid market (https://ecuagenera. net/products/lepanthes-deformis), potentially making it highly vulnerable to illegal collection. Limited studies on the conservation impacts of wild collection of epiphytic orchids suggest a low tolerance to harvesting (Fernández et al. 2003, Tremblay et al. 1998). The detrimental effects of unsustainable collection have been observed in other species, leading to reduced inflorescence development (Laelia autumnalis (Lex.) Lindl.; Emeterio-Lara et al. 2021), or even pushing them perilously close to extinction (Paphiopedilum canhii Aver. & O.Gruss; Averyanov et al. 2014). Although the full extent of the impact of illegal collection or other threats on Lepanthes populations is not fully understood, we recommend considering this species as a conservation concern as a precautionary measure until a comprehensive risk assessment is conducted (Brown et al. 2023).
Acknowledgements
The authors are grateful with the community of Virolin who have dedicated years studying their orchids, especially to ´Profe Mireya´, las Señoras Bertha, Lucia, and Adriana for their warm meals after fieldwork. We appreciate the support of Oscar Perez-Escobar, Robinson Galindo, David Haelterman, Holguer López, Andreas Kay and Melissa Alegria for kindly providing their images of the species referred to in this paper. In the same way, we want to thank Luis Baquero for his valuable input in the comprehension of some key differences of the new species. Instituto von Humboldt provided invaluable support, and especially to Mailyn Gonzalez and Jose Manuel Ochoa. We also thank the Editor, the anonymous reviewers for suggestions on the manuscript and many other colleagues who supported this work. This study was funded by the Natural Environment Research Council (grant no. NE/R017441/1). This is publication #46 of the Biodiversity, Agriculture, and Conservation in Colombia (Biodiversidad, Agricultura, y Conservación en Colombia [BACC]) project.
Author contributions. EPS: Writing - review & editing, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Fieldwork, ran the community workshops. ER: Taxonomic treatment, Writing - review & editing, Visualization and designing of Figures. YB, fieldwork and community leader. JCOB: Writing - review & editing. DPE: Conceptualization, Writing - review & editing, Supervision, Resources, Funding acquisition.
Funding. This study was funded by the Natural Environment Research Council (grant no. NE/R017441/1).
Conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Literature cited
-
Acevedo, M. A., Beaudrot, L., Meléndez-Ackerman, E. J. & Tremblay, R. L. (2020). Local extinction risk under climate change in a neotropical asymmetrically dispersed epiphyte. Journal of Ecology, 108(4), 1553-1564. https://doi.org/10.1111/1365-2745.13361
» https://doi.org/https://doi.org/10.1111/1365-2745.13361 - Averyanov, L. V., Pham Van The, Loc Phan Ke, Nguyen, H., Nguyen, T. V., Canh, C. X. & Nguyen, H. Q. (2014). Field survey of Pahiopedilum canhii: from discovery to extinction Slipper Orchids FALL, 2014, 2-11.
-
BGCI. (2023). ThreatSearch online database Botanic Gardens Conservation International Richmond, UK Retrieved from Retrieved from www.bgci.org/Threat_search.Php
Accessed 20 August 2023.
» www.bgci.org/Threat_search.Php -
Blanco, M. A. & Barboza, G. (2005). Pseudocopulatory pollination in Lepanthes (Orchidaceae: Pleurothallidinae) by fungus gnats. Annals of Botany, 95(5), 763-772. https://doi.org/10.1093/aob/mci090
» https://doi.org/https://doi.org/10.1093/aob/mci090 -
Bogarín, D., Pérez-Escobar, O. A., Groenenberg, D., Holland, S. D., Karremans, A. P., Lemmon, E. M., Lemmon, A. R., Pupulin, F., Smets, E. & Gravendeel, B. (2018). Anchored hybrid enrichment generated nuclear, plastid and mitochondrial markers resolve the Lepanthes horrida (Orchidaceae: Pleurothallidinae) species complex. Molecular Phylogenetics and Evolution, 129, 27-47. https://doi.org/10.1016/j.ympev.2018.07.014
» https://doi.org/https://doi.org/10.1016/j.ympev.2018.07.014 -
Brown, M. J. M., Bachman, S. P. & Nic Lughadha, E. (2023). Three in four undescribed plant species are threatened with extinction. New Phytologist, 240(4), 1340-1344. https://doi.org/10.1111/nph.19214
» https://doi.org/https://doi.org/10.1111/nph.19214 -
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., García, A., Pringle, R. M. & Palmer, T. M. (2015). Accelerated modern humaninduced species losses: Entering the sixth mass extinction. Science Advances, 1(5), e1400253. https://doi.org/10.1126/sciadv.1400253
» https://doi.org/https://doi.org/10.1126/sciadv.1400253 - Chase, M.W. & Peacor, D. (1987). Crystals of calcium oxalate hydrate on the perianth of Stelis Sw. Lindleyana, 2, 91-94.
-
Crain, B. J. & Tremblay, R. L. (2014). Do richness and rarity hotspots really matter for orchid conservation in light of anticipated habitat loss? Diversity and Distributions, 20(6), 652-662. https://doi.org/10.1111/ddi.12179
» https://doi.org/https://doi.org/10.1111/ddi.12179 -
Emeterio-Lara, A., García-Franco, J. G., Hernández-Apolinar, M., Toledo-Hernández, V. H., Valencia-Díaz, S. & Flores-Palacios, A. (2021). Does extraction of orchids affect their population structure? Evidence from populations of Laelia autumnalis (Orchidaceae). Forest Ecology and Management, 480, 118667. https://doi.org/10.1016/j.foreco.2020.118667
» https://doi.org/https://doi.org/10.1016/j.foreco.2020.118667 - Etter, A., Andrade, A., Saavedra, K., Amaya, P., Cortés, J. & Arévalo, P. (2021). Ecosistemas colombianos: amenazas y riesgos. Una aplicación de la Lista Roja de Ecosistemas a los ecosistemas terrestres continentales Pontificia Universidad Javeriana y Conservación Internacional-Colombia.
- Fernández, D. S., Tremblay, R. L., Ackerman, J. D., Rodríguez, E. & López, L. N. (2003). Reproductive potential, growth rate and light environment in Lepanthes rupestris Stimson. Lankesteriana, 7, 73-76.
-
Haddad, N. M., Brudvig, L. A., Clobert, J., Davies, K. F., González, A., Holt, R. D., Lovejoy, T. E., Sexton, J. O., Austin, M. P., Collins, C. D., Cook, W. M., Damschen, E. I., Ewers, R. M., Foster, B. L., Jenkins, C. N., King, A. J., Laurance, W. F., Levey, D. J., Margules, C. R., Melbourne, B. A., Nicholls, A. O., Orrock, J. L., Song, D.-X. & Townshend, J. R. (2015). Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science Advances, 1(2). https://doi.org/10.1126/sciadv.1500052
» https://doi.org/https://doi.org/10.1126/sciadv.1500052 -
Hinsley, A., de Boer, H. J., Fay, M. F., Gale, S. W., Gardiner, L. M., Gunasekara, R. S., Kumar, P., Masters, S., Metusala, D., Roberts, D. L., Veldman, S., Wong, S. & Phelps, J. (2018). A review of the trade in orchids and its implications for conservation. Botanical Journal of the Linnean Society, 186(4), 435-455. https://doi.org/10.1093/botlinnean/box083
» https://doi.org/https://doi.org/10.1093/botlinnean/box083 -
IUCN. (2020). The IUCN Red List of threatened species
v. 2019-3 Retrieved from https://www.iucnredlist.org.
» https://www.iucnredlist.org. -
Karremans, A. P., Moreno, J. S., Gil-Amaya, K., Gutiérrez Morales, N., Espinosa, F., Mesa, S., Restrepo, E., Rincón-González, M., Serna, A., Sierra-Ariza, M. & Vieira-Uribe, S. (2023). Colombian Orchidaceae: a catalogue of the Pleurothallidinae. Lankesteriana, 23(2), 181-400. https://doi.org/10.15517/lank.v23i2.56158
» https://doi.org/https://doi.org/10.15517/lank.v23i2.56158 -
Karremans, A. P., Rojas-Alvarado, G., Bezerra-Silva, L. E., Flores-Rojas, J., Murillo-Murillo, J. M., Romero-Ceciliano, M. & Segura-Bermúdez, O. A. (2021). Nomenclatural notes in the Pleurothallidinae (Orchidaceae): Miscellaneous part II. Phytotaxa, 496(2), 134-146. https://doi.org/10.11646/phytotaxa.496.2.3
» https://doi.org/https://doi.org/10.11646/phytotaxa.496.2.3 -
Kindlmann, P., Meléndez-Ackerman, E. J. & Tremblay, R. L. (2014). Disobedient epiphytes: colonization and extinction rates in a metapopulation of Lepanthes rupestris (Orchidaceae) contradict theoretical predictions based on patch connectivity. Botanical Journal of the Linnean Society, 175(4), 598-606. https://doi.org/10.1111/boj.12180
» https://doi.org/https://doi.org/10.1111/boj.12180 - Luer, C. A. (1990). Icones Pleurothallidinarum VII. Systematics of Platystele (Orchidaceae). Monographs in Systematic Botany from the Missouri Botanical Garden, 38, 1-132.
- Luer, C. A. (1996). Icones Pleurothallidinarum. XIV. The genus Lepanthes, subgenus Lepanthes in Ecuador. Monographs in Systematic Botany from the Missouri Botanical Garden, 61(3), 1-255.
- Luer, C. A. & Hirtz, A. C. (1987). New species of Lepanthes Die Orchidee, 38(1), 38.
- Luer, C. A. & Thoerle, L. (2012). Icones Pleurothallidinarum. XXXII. Lepanthes of Colombia (Orchidaceae). Monographs in Systematic Botany from the Missouri Botanical Garden, 123, 1-300.
-
Moreno, J. S., Sandoval-Arango, S., Palacio, R. D., Alzate, N. F., Rincón, M., Gil, K., Morales, N. G., Harding, P. & Hazzi, N. A. (2020). Distribution Models and Spatial Analyses Provide Robust Assessments of Conservation Status of Orchid Species in Colombia: The Case of Lephantes mucronata Harvard Papers in Botany, 25(1), 111-121. https://doi.org/10.3100/hpib.v25iss1.2020.n14
» https://doi.org/https://doi.org/10.3100/hpib.v25iss1.2020.n14 -
Parra-Sánchez, E., Pérez-Escobar, O. A. & Edwards, D. P. (2023a). Neutral-based processes overrule niche-based processes in shaping tropical montane orchid communities across spatial scales. Journal of Ecology, 111(8), 1614-1628. https://doi.org/10.1111/1365-2745.14140
» https://doi.org/https://doi.org/10.1111/1365-2745.14140 -
Parra-Sánchez, E., Restrepo, E., Alegria-Valencia, M., Moreno, J. S. & Galindo-Tarazona, R. (2023b). Lepanthes cordillerana (Orchidaceae) a new species and the landscape threats to its wild populations. Phytotaxa, 603(1), 69-80. https://doi.org/10.11646/phytotaxa.603.1.5
» https://doi.org/https://doi.org/10.11646/phytotaxa.603.1.5 -
Parra-Sánchez, E., Vieira-Uribe, S. & Moreno, J. S. (2023c). new species of Lepanthes (Orchidaceae: Pleurothallidinae) to honour “Bachué”, the mythological mother of the indigenous Muisca people. Lankesteriana, 23(2), 127-137. https://doi.org/10.15517/lank.v23i2.54032
» https://doi.org/https://doi.org/10.15517/lank.v23i2.54032 -
Pérez-Escobar, O. A., Chomicki, G., Condamine, F. L., Karremans, A. P., Bogarín, D., Matzke, N. J., Silvestro, D. & Antonelli, A. (2017). Recent origin and rapid speciation of Neotropical orchids in the world’s richest plant biodiversity hotspot. New Phytologist, 215(2), 891-905. https://doi.org/10.1111/nph.14629
» https://doi.org/https://doi.org/10.1111/nph.14629 -
QGIS. (2021). QGIS Geographic Information System QGIS Association. Retrieved from QGIS.org
» QGIS.org - Rabinowitz, D. (1981). Seven forms of rarity. In H. Synge (Ed.), The biological aspects of rare plant conservation (pp. 205-217). John Wiley & Sons.
- Romero-González, G.A. & Carnevali, G. (2000). Orquídeas de Venezuela [una Guía de Campo Ilustrada]. Caracas: Armitano Editores.
- Stearn, W. T. (1992). Botanical Latin (4th ed.). Portland, USA: Timber Press
-
Tadono, T., Ishida, H., Oda, F., Naito, S., Minakawa, K. & Iwamoto, H. (2014). Precise Global DEM Generation by ALOS PRISM. ISPRS Annals of the Photogrammetry, Remote Sensing and Spatial Information Sciences, II-4, 71-76. https://doi.org/10.5194/isprsannals-II-4-71-2014
» https://doi.org/https://doi.org/10.5194/isprsannals-II-4-71-2014 -
Travis, J. M. J. (2003). Climate change and habitat destruction: a deadly anthropogenic cocktail. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1514), 467-473. https://doi.org/10.1098/rspb.2002.2246
» https://doi.org/https://doi.org/10.1098/rspb.2002.2246 - Tremblay, R. L. (1997). Distribution and Dispersion Patterns of Individuals in Nine Species of Lepanthes (Orchidaceae). Biotropica, 29(1), 38-45.
-
Tremblay, R. L., Zimmerman, J. K., Lebrón, L., Bayman, P., Sastre, I., Axelrod, F. & Alers-García, J. (1998). Host specificity and low reproductive success in the rare endemic Puerto Rican orchid Lepanthes caritensis Biological Conservation, 85(3), 297-304. https://doi.org/10.1016/S0006-3207(97)00163-8
» https://doi.org/https://doi.org/10.1016/S0006-3207(97)00163-8 -
Vancutsem, C., Achard, F., Pekel, J.-F., Vieilledent, G., Carboni, S., Simonetti, D., Gallego, J., Aragão, L. E. O. C. & Nasi, R. (2021). Long-term (1990-2019) monitoring of forest cover changes in the humid tropics. Science Advances, 7(10), 1-21. https://doi.org/10.1126/sciadv.abe1603
» https://doi.org/https://doi.org/10.1126/sciadv.abe1603 -
Wilson, A. M. & Jetz, W. (2016). Remotely Sensed High-Resolution Global Cloud Dynamics for Predicting Ecosystem and Biodiversity Distributions. PLOS Biology, 14(3), e1002415. https://doi.org/10.1371/journal.pbio.1002415
» https://doi.org/https://doi.org/10.1371/journal.pbio.1002415 -
Zizka, A., Silvestro, D., Vitt, P. & Knight, T. M. (2021). Automated conservation assessment of the orchid family with deep learning. Conservation Biology, 35(3), 897-908. https://doi.org/10.1111/cobi.13616
» https://doi.org/https://doi.org/10.1111/cobi.13616
Publication Dates
-
Date of issue
May-Aug 2024
History
-
Received
12 Mar 2024 -
Accepted
12 June 2024

 Lepanthes chalalensis (pleurothallidinae), a new species endemic to the santander department in Colombia
Lepanthes chalalensis (pleurothallidinae), a new species endemic to the santander department in Colombia




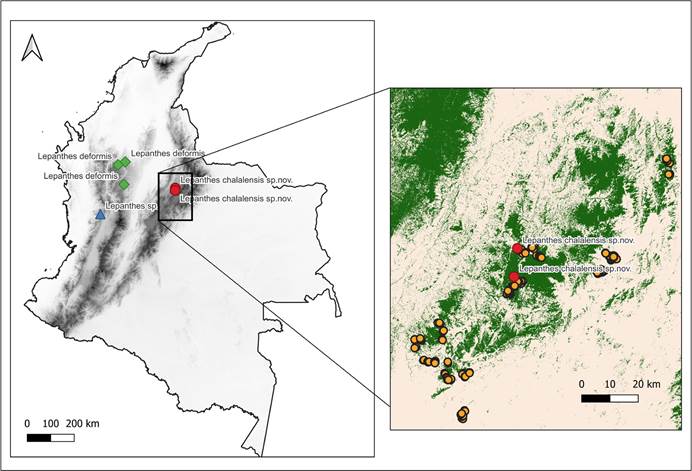 Map composed by E.Parra-Sánchez using QGis 3.241. Left panel shows the distribution of Lepanthes chalalensis (red dots), L. deformis Luer & Hirtz (green dots), and Lepanthes sp. as aff. deformis (blue triangle; Pérez-Escobar O., pers. comm.). Right panel displays study area within Colombia (black box), and elevation (digital elevation model from
Map composed by E.Parra-Sánchez using QGis 3.241. Left panel shows the distribution of Lepanthes chalalensis (red dots), L. deformis Luer & Hirtz (green dots), and Lepanthes sp. as aff. deformis (blue triangle; Pérez-Escobar O., pers. comm.). Right panel displays study area within Colombia (black box), and elevation (digital elevation model from 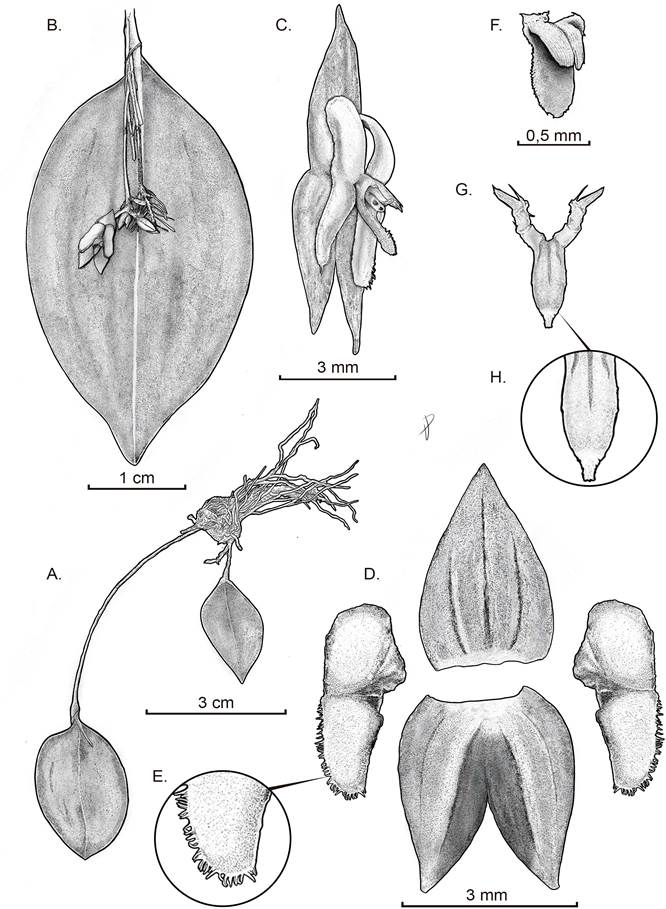 Drawn by Daniel Amaya-Jiménez from the plant that served as type. A. Habit. B. Abaxial view of the leaf with flower. C. Flower. D. Dissected perianth. E. Zoom of the petal’s lobe. F. Lip in natural position – view. G. Lip in natural position without column front view and lip expanded. H. Zoom on the lip’s apex.
Drawn by Daniel Amaya-Jiménez from the plant that served as type. A. Habit. B. Abaxial view of the leaf with flower. C. Flower. D. Dissected perianth. E. Zoom of the petal’s lobe. F. Lip in natural position – view. G. Lip in natural position without column front view and lip expanded. H. Zoom on the lip’s apex.
 Photographed and prepared by Eugenio Restrepo from the plant that served as type (Parra-Sanchez 2401, VALLE). A. Leaf plus flower in abaxial view. B. Flower, frontal view. C. Flower, lateral view. D. Dissected perianth. E. Plant habit.
Photographed and prepared by Eugenio Restrepo from the plant that served as type (Parra-Sanchez 2401, VALLE). A. Leaf plus flower in abaxial view. B. Flower, frontal view. C. Flower, lateral view. D. Dissected perianth. E. Plant habit.
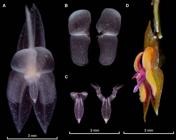
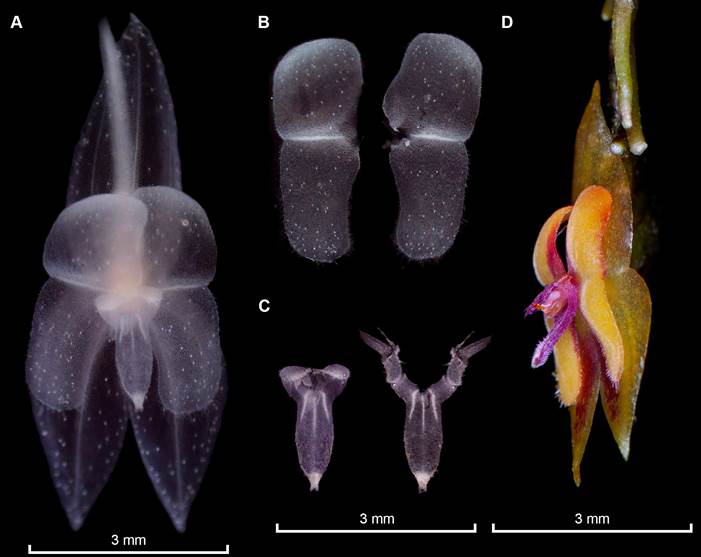 Photographs by Melisa Alegría-Valencia (A-C) and Holguer López (D). Prepared by Eugenio Restrepo from the plant that served as type, except D (Parra-Sanchez 2401, VALLE). A. Entire flower preserved in spirit showing the “silver-like crystals” present in sepals, petals, column, and lip. B. Frontal view of the petals fully extended, note that the long cilia that can be seen in-vivo are not striking in the image. C. Frontal view of the lip, at the left the laminae not extended and extended at the right depicting the long sinus, and laminae extended. D. Flower in semilateral view showing the relation between the appendix and the lip length.
Photographs by Melisa Alegría-Valencia (A-C) and Holguer López (D). Prepared by Eugenio Restrepo from the plant that served as type, except D (Parra-Sanchez 2401, VALLE). A. Entire flower preserved in spirit showing the “silver-like crystals” present in sepals, petals, column, and lip. B. Frontal view of the petals fully extended, note that the long cilia that can be seen in-vivo are not striking in the image. C. Frontal view of the lip, at the left the laminae not extended and extended at the right depicting the long sinus, and laminae extended. D. Flower in semilateral view showing the relation between the appendix and the lip length.
 Photographs by Robinson Galindo-Tarazona (A), David Haelterman (B), and Andreas Kay (C). Prepared by Eugenio Restrepo. A. Lepanthes chalalensis E.Restrepo & E.Parra. B. Lepanthes velosa Luer & Hirtz. C. Lepanthes deformis Luer & Hirtz.
Photographs by Robinson Galindo-Tarazona (A), David Haelterman (B), and Andreas Kay (C). Prepared by Eugenio Restrepo. A. Lepanthes chalalensis E.Restrepo & E.Parra. B. Lepanthes velosa Luer & Hirtz. C. Lepanthes deformis Luer & Hirtz.